Table 2.
Stereoselective synthesis of (2,3-syn-3,4-syn-4,5-syn-5,6-anti)-1-aza-7-oxabicyclo[2.2.1]heptanes.
 | |||||
|---|---|---|---|---|---|
| entry | homoallylic hydroxylamine | R3CHO | yield (%) | dr | product |
| 1 |
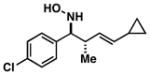 34 |
|
61 | ≥20:1 |
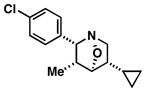 35 |
| 2 |
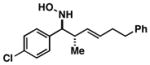 36 |
|
60 | ≥20:1 |
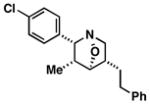 37 |
| 3 |
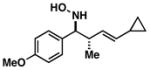 38 |
|
66 | 6:1a |
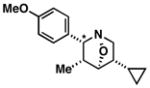 39 |
| 4 |
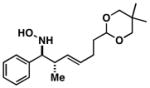 40 |
|
61 | 6:1a |
 41 |
| 5 |
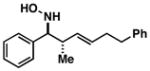 29 |
|
80 | 4:1 |
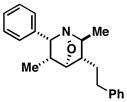 42 |
= minor product is epimeric at the highlighted (*) position.
Note: Initial sigmatropic rearrangement for reactions summarized in entries 1 and 2 occur in <2 h, whereas substrates depicted in entries 3 and 4 required up to 48 h. Similarly, the subsequent [3+2]annulation in entries 3 and 4 require prolonged heating in comparison to entries 1 and 2 (48 h at 120 °C vs. 12 h at 120 °C). Note: products are racemic.
