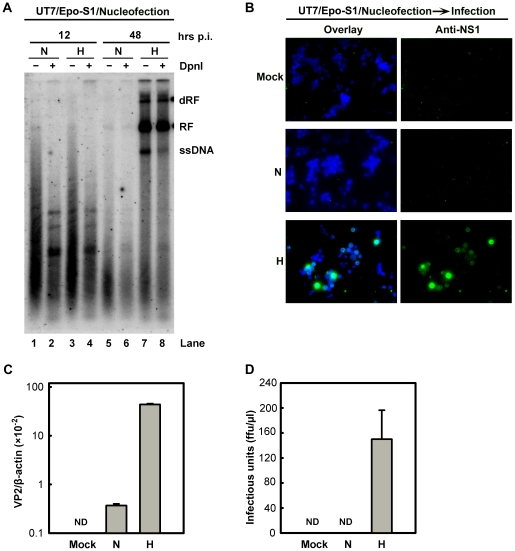
Figure 4. B19V DNA replication and de novo production of infectious virions in UT7/Epo-S1 cells under hypoxia.
(A) The rate of replication of transfected B19V infectious DNA (M20) was measured in UT7/Epo-S1 cells under hypoxia (H) vs. normoxia (N). UT7/Epo-S1 cells were pre-cultured under hypoxia for 2 days prior to nucleofection with the M20 DNA. At the times indicated, Hirt DNA was prepared, digested with (+) or without (−) Dpn I, and analyzed by Southern blotting. dRF, RF and ssDNA bands are indicated. (B, C&D) B19V infectious virions produced from nucleofection of the M20 DNA into UT7/Epo-S1 cells was boosted under hypoxia. UT7/Epo-S1 cells cultured under hypoxia (H) or normoxia (N) were nucleofected with the M20 DNA. At 48 hrs post-nucleofection, the cells were pelleted and resuspended in CD36+ EPC expansion medium followed by three cycles of freeze-thaw and a briefly spin. This virus preparation was used to infect hypoxia-cultured CD36+ EPCs. At 48 hrs p.i., immunofluorescence staining was performed using anti-NS1 (B), images were acquired at a magnification of 20×(objective lens) using an Eclipse Ti-S inverted microscope (Nikon) controlled by MetaMorph software (Molecular Devices); and the levels of VP2-encoding mRNA per β-actin mRNA were quantified (C). Yields of infectious virions in ffu were calculated (D). ND, not detectable.