Abstract
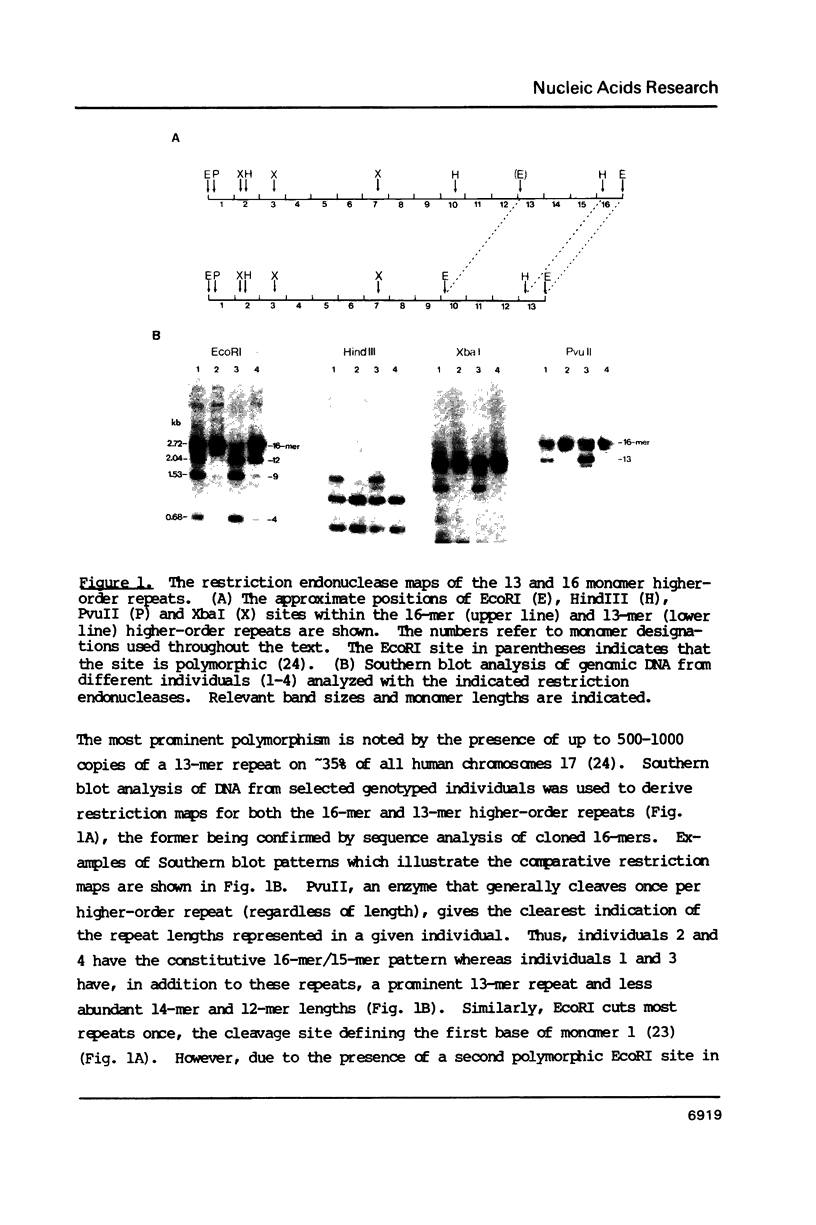
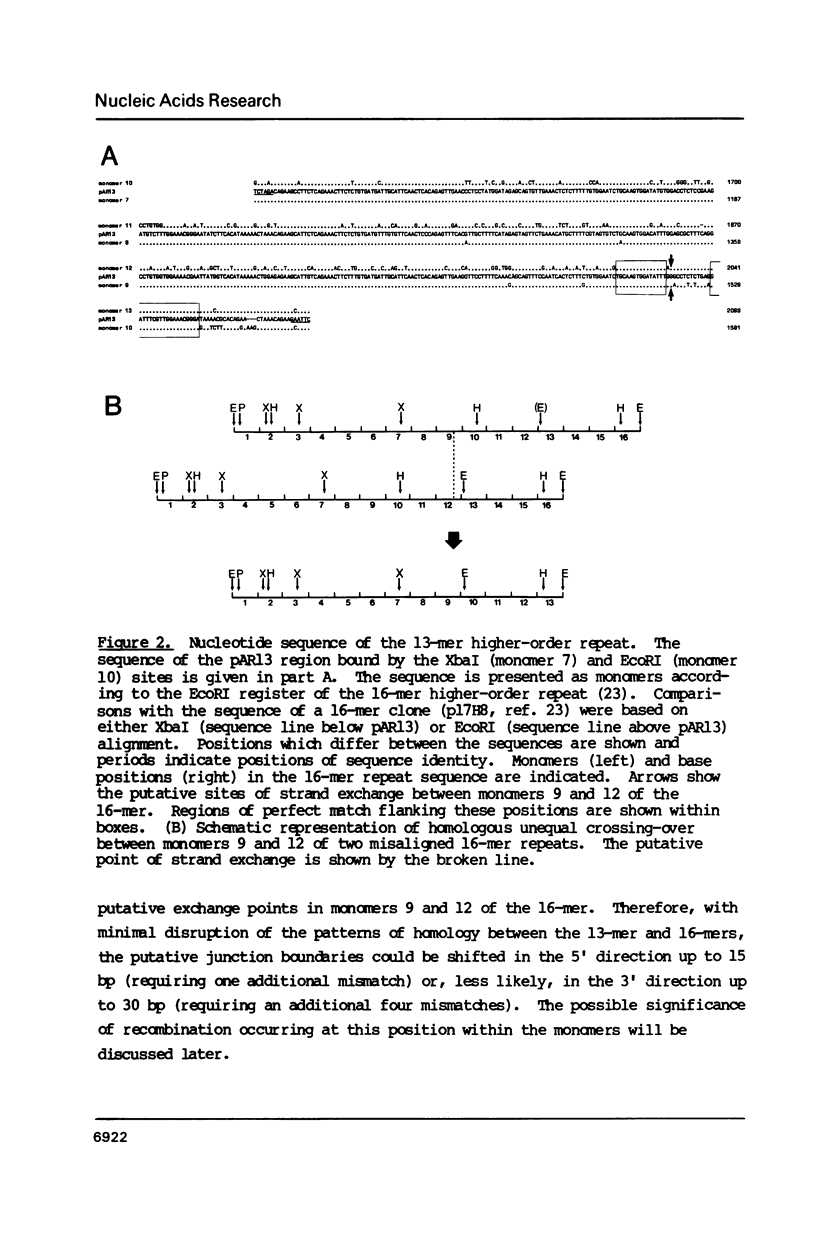
The human alpha satellite DNA family is organized into chromosome-specific subsets characterized by distinct higher-order repeats based on a approximately 171 basepair monomer unit. On human chromosome 17, the predominant form of alpha satellite is a 16-monomer (16-mer) higher-order repeat present in 500-1000 copies per chromosome 17. In addition, less abundant 15-monomer and 14-monomer repeats are also found constitutively on chromosome 17. Polymorphisms in the form of different higher-order repeat lengths have been described for this subset, the most prominent polymorphism being a 13-monomer (13-mer) higher-order repeat present on approximately 35% of all chromosomes 17. To investigate the nature of this polymorphism, we have cloned, sequenced and compared the relevant regions of the 13-mer to the previously characterized 16-mer repeat. The results show that the repeats are virtually identical, with the principal difference being the exclusion of three monomers from the 13-mer repeat. We propose that the 13-mer is the product of an isolated homologous recombination event between two monomers of the 16-mer repeat. Sequence comparisons reveal the approximate site of recombination and flanking regions of homology. This recombination site corresponds to a position within the alphoid monomer which has been previously implicated in an independent homologous recombination event, suggesting that there may exist a preferred register for recombination in alphoid DNA. We suggest that these events are representative of an ongoing process capable of reorganizing the satellite subset of a given chromosome, thereby contributing to the establishment of chromosome-specific alpha satellite subsets.
Full text
PDF





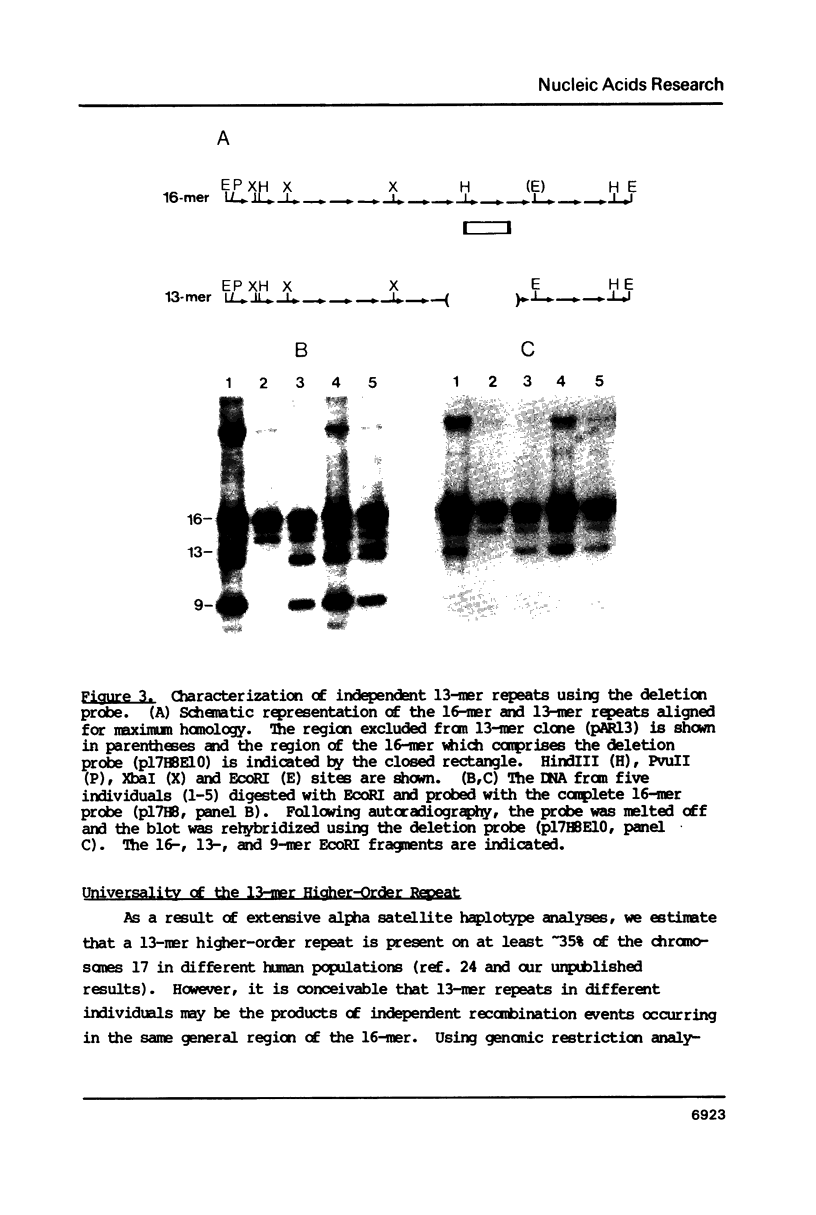




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Brown F. L., Musich P. R., Maio J. J. Cae I: an endonuclease isolated from the African green monkey with properties indicating site-specific cleavage of homologous and heterologous mammalian DNA. Nucleic Acids Res. 1978 Apr;5(4):1093–1107. doi: 10.1093/nar/5.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D. L. Molecular arrangement and evolution of heterochromatic DNA. Annu Rev Genet. 1980;14:121–144. doi: 10.1146/annurev.ge.14.120180.001005. [DOI] [PubMed] [Google Scholar]
- Donehower L., Gillespie D. Restriction site periodicities in highly repetitive DNA of primates. J Mol Biol. 1979 Nov 15;134(4):805–834. doi: 10.1016/0022-2836(79)90487-x. [DOI] [PubMed] [Google Scholar]
- Dover G. Molecular drive: a cohesive mode of species evolution. Nature. 1982 Sep 9;299(5879):111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V. On spacers. Cell. 1979 Apr;16(4):697–710. doi: 10.1016/0092-8674(79)90086-2. [DOI] [PubMed] [Google Scholar]
- Gillespie D. Newly evolved repeated DNA sequences in primates. Science. 1977 May 20;196(4292):889–891. doi: 10.1126/science.870965. [DOI] [PubMed] [Google Scholar]
- Jørgensen A. L., Bostock C. J., Bak A. L. Chromosome-specific subfamilies within human alphoid repetitive DNA. J Mol Biol. 1986 Jan 20;187(2):185–196. doi: 10.1016/0022-2836(86)90227-5. [DOI] [PubMed] [Google Scholar]
- Kedes L. H. Histone genes and histone messengers. Annu Rev Biochem. 1979;48:837–870. doi: 10.1146/annurev.bi.48.070179.004201. [DOI] [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Gravel R. A. Rapid and reliable dideoxy sequencing of double-stranded DNA. Gene. 1985;40(2-3):317–323. doi: 10.1016/0378-1119(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: recurrent periodicities and models for the evolutionary origins of repetitive DNA. J Mol Biol. 1977 Dec 15;117(3):637–655. doi: 10.1016/0022-2836(77)90062-6. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Toward a molecular paleontology of primate genomes. I. The HindIII and EcoRI dimer families of alphoid DNAs. Chromosoma. 1981;83(1):103–125. doi: 10.1007/BF00286019. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Wu J. C. Homology between human and simian repeated DNA. Nature. 1978 Nov 2;276(5683):92–94. doi: 10.1038/276092a0. [DOI] [PubMed] [Google Scholar]
- McKenna W. G., Brown F. L., Musich P. R., Maio J. J. Cleavage of mammalian repetitive deoxyribonucleic acids by a mammalian site-specific endodeoxyribonuclease. J Mol Biol. 1982 Jan 15;154(2):379–384. doi: 10.1016/0022-2836(82)90070-5. [DOI] [PubMed] [Google Scholar]
- McKenna W. G., Maio J. J., Brown F. L. Purification and properties of a mammalian endonuclease showing site-specific cleavage of DNA. J Biol Chem. 1981 Jun 25;256(12):6435–6443. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich P. R., Brown F. L., Maio J. J. Nucleosome phasing and micrococcal nuclease cleavage of African green monkey component alpha DNA. Proc Natl Acad Sci U S A. 1982 Jan;79(1):118–122. doi: 10.1073/pnas.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich P. R., Maio J. J., Brown F. L. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: indications of a phase relation between restriction sites and chromatin subunits in African green monkey and calf nuclei. J Mol Biol. 1977 Dec 15;117(3):657–677. doi: 10.1016/0022-2836(77)90063-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Singer M., Rosenberg M. Highly reiterated sequences of SIMIANSIMIANSIMIANSIMIANSIMIAN. Science. 1978 Apr 28;200(4340):394–402. doi: 10.1126/science.205944. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Deininger P. L., Houck C. M., Schmid C. W. A dimer satellite sequence in bonnet monkey DNA consists of distinct monomer subunits. J Mol Biol. 1980 Jan 15;136(2):151–167. doi: 10.1016/0022-2836(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. Highly repeated sequences in mammalian genomes. Int Rev Cytol. 1982;76:67–112. doi: 10.1016/s0074-7696(08)61789-1. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Chromosome-specific alpha satellite DNA: nucleotide sequence analysis of the 2.0 kilobasepair repeat from the human X chromosome. Nucleic Acids Res. 1985 Apr 25;13(8):2731–2743. doi: 10.1093/nar/13.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F. Chromosome-specific organization of human alpha satellite DNA. Am J Hum Genet. 1985 May;37(3):524–532. [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. C., Manuelidis L. Sequence definition and organization of a human repeated DNA. J Mol Biol. 1980 Sep 25;142(3):363–386. doi: 10.1016/0022-2836(80)90277-6. [DOI] [PubMed] [Google Scholar]
- Wu K. C., Strauss F., Varshavsky A. Nucleosome arrangement in green monkey alpha-satellite chromatin. Superimposition of non-random and apparently random patterns. J Mol Biol. 1983 Oct 15;170(1):93–117. doi: 10.1016/s0022-2836(83)80228-9. [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Fittler F., Hörz W. Eight different highly specific nucleosome phases on alpha-satellite DNA in the African green monkey. Nucleic Acids Res. 1983 Jul 11;11(13):4287–4306. doi: 10.1093/nar/11.13.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]