Abstract
Background
To compare tumor volume and patient weight vs. traditional factors of tumor diameter and patient age, to determine which parameters best discriminates outcome among intermediate risk RMS patients.
Methods
Complete patient information for non-metastatic RMS patients enrolled in the Children’s Oncology Group (COG) intermediate risk study D9803 (1999–2005) was available for 370 patients. The Kaplan-Meier method was used to estimate survival distributions. A recursive partitioning model was used to identify prognostic factors associated with event-free survival (EFS). Cox-proportional hazards regression models were used to estimate the association between patient characteristics and the risk of failure or death.
Results
For all intermediate risk patients with RMS, a recursive partitioning algorithm for EFS suggests that prognostic groups should optimally be defined by tumor volume (transition point 20 cm3), weight (transition point 50 kg), and embryonal histology. Tumor volume and patient weight added significant outcome information to the standard prognostic factors including tumor diameter and age (p=0.02). The ability to resect the tumor completely was not significantly associated with the size of the patient, and patient weight did not significantly modify the association between tumor volume and EFS after adjustment for standard risk factors (p=0.2).
Conclusion
The factors most strongly associated with EFS were tumor volume, patient weight, and histology. Based on regression modeling, volume and weight are superior predictors of outcome compared to tumor diameter and patient age in children with intermediate risk RMS. Prognostic performance of tumor volume and patient weight should be assessed in an independent prospective study.
Keywords: rhabdomyosarcoma, prognosis, prognostic factors, tumor volume, patient size
INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common malignant soft-tissue tumor of childhood. Through clinical trials utilizing multimodality therapy, survival has steadily improved over the last three decades.1 Patients are stratified into a low, intermediate or high risk category based on the clinical Group (completeness of surgical resection at presentation), pre-treatment TNM staging classification (site and size of primary tumor, tumor invasion into surrounding tissues, nodal status, and presence of metastatic disease), and tumor histology.2 These prognostic factors, as well as age, predict outcome for intermediate risk tumors.3
For sarcomas, 5 cm has been used as the transition point to predict outcome and determine disease risk stratification; tumor size >5cm is associated with a worse prognosis. In addition, patient age <1 or >10 years are also associated with a worse prognosis.1, 4–6 Although RMS occurs in patients of varied ages and body sizes, the size of the tumor is characterized by maximum single dimension and the pre-treatment TNM staging does not take into account the relationship between tumor diameter and the patients’ age or body size. Ferrari et al. recently evaluated the association between tumor diameter and the patient body size, quantified by body surface area, and correlated these factors with outcomes in childhood soft tissue sarcomas (STS).7 In localized RMS, for a given tumor diameter, the mortality rate was higher for those patients with a lower body surface area. These data suggest that it is not the absolute diameter of the tumor but the relationship between tumor diameter and body surface area that may be a superior discriminator of outcome. In a subsequent study they demonstrated that both tumor volume and diameter measurements at presentation were equally predictive of overall survival as well as response to induction chemotherapy.8
Based on this background we compared the role of tumor volume and patient weight vs. tumor diameter and patient age, as prognostic factors in intermediate risk RMS. In addition, we determined whether tumor volume relative to patient weight was more predictive of outcome than tumor volume.
METHODS
Patient Population
Children’s Oncology Group (COG) study D9803 enrolled 570 intermediate risk RMS patients from 1999 to 2005 and was used for this study. Patients eligible for this analysis had non-metastatic alveolar RMS or non-metastatic embryonal RMS arising in unfavorable sites. The overall results for COG D9803 have been previously reported.9 Tumor volume measurements or other data values were missing for 200 subjects, leaving 370 patients for further evaluation. The tumor volume and tumor diameter measurements were obtained from diagnostic imaging studies in 96% of analyzed subjects and from physical exam measurements in 4% of subjects. Informed consent was obtained by the study participants. Tumor volume was calculated using software associated with the institutions imaging technique but in general was the product of the maximum perpendicular diameters of the largest primary tumor whereas tumor diameter was defined as the maximum diameter of the largest primary tumor.
Statistical Method
The distributions of categorical subject characteristics were compared among independent groups using a Chi-square test, or Fisher’s exact test when expected cell counts were small. The Kruskal-Wallis test was used to compare the distributions of continuous measures among independent groups. Event-free survival (EFS) was defined as the time from study enrollment to disease progression or death. EFS for patients who had not experienced an event were censored at the patient’s last date of contact. The Kaplan-Meier method was used to estimate the survival distribution for groups of patients defined by patient or disease characteristics and the distributions were compared using a log-rank test. In order to identify risk groups, a recursive partitioning method was used.10, 11 Noting that some of the subgroups splits may overfit the data, a graphical approach is used to prune off splits that are not necessary.11 Some of the subgroups in the resulting tree may have similar survival experiences and were combined by using the recursive partitioning algorithm based on the prognostic group identifiers. The recursive partitioning approach results in more easily interpretable prognostic groups, defined by higher order interactions, than the Cox-proportional hazard regression modeling approach, and has been used in the oncology setting.10–12 Cox-proportional hazard regression models were used to estimate the hazard of an event or death associated with risk groups defined using cut-points suggested by the recursive partitioning approach rounded to the nearest 5 kilograms for weight, 1 centimeter for diameter, or 5 cubic centimeters for volume for ease of clinical interpretation. The change in the likelihood ratio test statistic was used to determine if additional subject characteristics were associated with the hazard of an event beyond the identified risk group factors. Data were current through June 2008, and the median follow-up was 4.4 years (range 0.1 to 8.2 years).
RESULTS
Patient Characteristics
Patient characteristics are summarized in Table 1. The subjects analyzed in this study (n=370) differed significantly from the subjects missing tumor volume measurements (n=190) or other clinical data (n=10) in terms of stage, group, primary site, tumor size, tumor invasiveness, lymph node involvement, histology, and risk stratification. There were more patients with advanced disease in the group with complete information that were utilized for the tumor volume analysis. However, even with these differences the 4-year EFS was estimated to be 0.71 (95% CI: 0.66–0.75) among the subjects analyzed in this study (n=370), which is similar to subjects excluded from this study due to missing tumor volume measurements or other clinical data (n=200, 4-year EFS = 0.71, 95% CI: 0.64 to 0.77) and the entire cohort (n=570, 4-year EFS = 0.71, 95% CI: 0.67–0.75).
Table 1.
Demographic and Disease Characteristics for Subjects Included (n=370) and Excluded (n=200) from the Analysis Set.
| Subjects with Tumor Volume Measures and Complete Covariate Information (n=370) | Subjects Missing Tumor Volume or Other Covariate Data (n=200) | P-value | |
|---|---|---|---|
| Count (column %) | Count (column %) | ||
| Age (years) | 0.3 | ||
| <1 | 10 (3%) | 5 (3%) | |
| 1–9 | 255 (69%) | 126 (63%) | |
| 10+ | 105 (28%) | 69 (35%) | |
| Sex | 0.2 | ||
| Male | 224 (61%) | 133 (67%) | |
| Female | 146 (39%) | 67 (34%) | |
| Stage | <0.0001 | ||
| 1 | 35 ( 9%) | 49 (26%) | |
| 2 | 104 (28%) | 53 (28%) | |
| 3 | 231 (62%) | 88 (46%) | |
| Group | <0.0001 | ||
| I | 12 ( 3%) | 20 (11%) | |
| II | 32 ( 9%) | 45 (24%) | |
| III | 326 (88%) | 125 (66%) | |
| Primary Site | <0.0001 | ||
| Extremity | 68 (18%) | 21 (11%) | |
| GU/BP | 62 (17%) | 20 (11%) | |
| Parameningeal | 140 (38%) | 66 (35%) | |
| Retroperitoneal/Perineal | 49 (13%) | 20 (11%) | |
| Other | 51 (14%) | 62 (33%) | |
| Tumor Size | 0.003 | ||
| ≤ 5 cm | 146 (39%) | 99 (53%) | |
| >5 cm | 224 (61%) | 88 (47%) | |
| Tumor Invasion | 0.04 | ||
| T-1 | 172 (46%) | 103 (55%) | |
| T-2 | 198 (54%) | 84 (45%) | |
| Regional Lymph Nodes | 0.02 | ||
| N-0 | 309 (84%) | 143 (76%) | |
| N-1 | 61 (16%) | 45 (24%) | |
| Histology | 0.0001 | ||
| Embryonal | 183 (49%) | 62 (32%) | |
| Alveolar | 159 (43%) | 116 (60%) | |
| Undifferentiated | 8 (2%) | 9 ( 5%) | |
| RMS, NOS | 20 (5%) | 6 ( 3%) | |
| Strata (composite) | <0.0001 | ||
| Embryonal Stage 2/3 Group III | 157 (42%) | 46 (23%) | |
| ALV/UDS Stage 1 or Group I | 41 (11%) | 64 (32%) | |
| ALV/UDS other | 108 (29%) | 60 (30%) | |
| PM with extension | 64 (17%) | 30 (15%) |
Prognostic Significance of Tumor Volume and Patient Weight
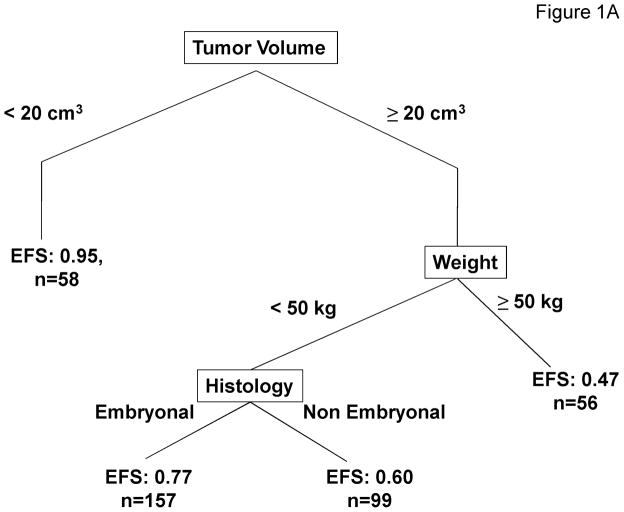
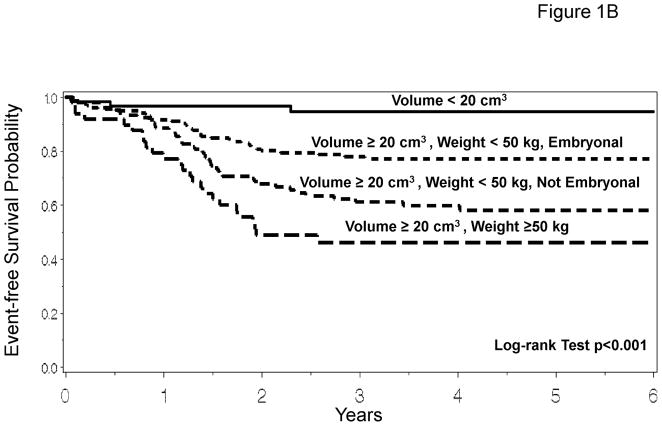
The recursive partitioning algorithm considered the following variables: age, stage, group, T-stage, nodal status, histology, primary site, maximum tumor diameter, tumor volume, patient height, patient weight, body surface area, and treatment regimens. Based on the recursive partitioning algorithm, when considering all variables including tumor volume and patient weight, the best predictor of outcome was tumor volume (transition point 20 cm3). Among subjects with tumor volumes ≥20 cm3, the model suggests splitting the subjects into groups depending on weight (transition point 50 kg). Finally, subjects with tumor volumes ≥20 cm3 and weight < 50 kg are stratified based on tumor histology (embryonal or non-embryonal). The recursive partitioning model results in 4 risk groups: (1) subjects with tumor volume <20 cm3 [n=58, 4-year EFS =0.95 with 95% CI: 0.84–0.98]; (2) subjects with tumor volume ≥20 cm3 and weight < 50 kg with embryonal disease [n=157, 4-year EFS=0.77 with 95% CI: 0.69–0.83]; (3) subjects with tumor volume ≥20 cm3 and weight < 50 kg with non-embryonal disease [n=99, 4-year EFS =0.60 with 95% CI: 0.49–0.69]; and (4) subjects with tumor volume ≥20 cm3 and weight ≥50 kg [n=56, 4-year EFS =0.47 with 95% CI: 0.33–0.60] (Figure 1A). EFS Kaplan-Meier curves for these risk groups are presented in Figure 1B, showing the prognostic utility of tumor volume and patient weight separating patients into distinct risk groups (p<0.001, overall log-rank test). Disease and patient characteristics for each risk stratification group are described in Table 2. Characteristics related to the factors used to define the risk groups, patient age, tumor stage, tumor histology and tumor size, are significantly different among the risk groups. In addition, other standard prognostic factors also differed significantly among the 4 risk groups.
Figure 1.
Prognostic Significance of Tumor Volume and Patient Weight. 1A. Recursive partitioning algorithm utilizing the following variables as potential prognostic factors for EFS: age, stage, group, T-stage, nodal status, histology, primary site, maximum tumor diameter, tumor volume, patient height, patient weight, patient body surface area, and treatment regimens. 1B. EFS Kaplan-Meier curves for risk groups identified by the recursive partitioning.
Table 2.
Subject Characteristics for Risk Groups Using Tumor Volume and Patient Weight.
| Risk Group 1 (Tumor volume < 20 cm3) (n=58) | Risk Group 2 (Tumor volume ≥20 cm3, weight < 50 kg, embryonal) (n=157) | Risk Group 3 (Tumor volume ≥ 20 cm3, weight < 50 kg, non-embryonal) (n=99) | Risk Group 4 (Tumor volume ≥ 20 cm3, weight ≥ 50 kg) (n=56) | p-value* (overall) | |
|---|---|---|---|---|---|
| Count (%) | Count (%) | Count (%) | Count (%) | ||
| Age (years) | <0.001 | ||||
| <1 | 1 (2%) | 4 (3%) | 5 (5%) | 0 | |
| 1–9 | 41 (71%) | 138 (88%) | 75 (76%) | 1 (2%) | |
| 10+ | 16 (28%) | 15 (10%) | 19 (19%) | 55 (98%) | |
| Stage | <0.001 | ||||
| 1 | 14 (24%) | 0 | 16 (16%) | 5 ( 9%) | |
| 2 | 34 (59%) | 37 (24%) | 24 (24%) | 9 (16%) | |
| 3 | 10 (17%) | 120 (76%) | 59 (60%) | 42 (75%) | |
| Group | <0.001 | ||||
| I | 7 (12%) | 0 | 4 (4%) | 1 (2%) | |
| II | 9 (16%) | 2 (1%) | 17 (17%) | 4 ( 7%) | |
| III | 42 (72%) | 155 (99%) | 78 (79%) | 51 (91%) | |
| Primary Site | <0.001 | ||||
| Extremity | 13 (22%) | 15 (10%) | 30 (30%) | 10 (18%) | |
| GU/BP | 6 (10%) | 42 (27%) | 8 (8%) | 6 (11%) | |
| Parameningeal | 22 (38%) | 62 (39%) | 28 (28%) | 28 (50%) | |
| Retroperitoneal/Perineal | 0 | 33 (21%) | 11 (11%) | 5 ( 9%) | |
| Other | 17 (29%) | 5 (3%) | 22 (22%) | 7 (13%) | |
| Tumor Size (diameter) | <0.001 | ||||
| ≤ 5 cm | 55 (95%) | 40 (25%) | 35 (35%) | 16 (29%) | |
| >5 cm | 3 (5%) | 117 (75%) | 64 (65%) | 40 (71%) | |
| Tumor Invasion | 0.002 | ||||
| T-1 | 36 (62%) | 64 (41%) | 54 (55%) | 18 (32%) | |
| T-2 | 22 (38%) | 93 (59%) | 45 (45%) | 38 (68%) | |
| Regional Lymph Nodes | <0.001 | ||||
| N-0 | 49 (84%) | 142 (90%) | 83 (84%) | 35 (63%) | |
| N-1 | 9 (16%) | 15 (10%) | 16 (16%) | 21 (38%) | |
| Histology | -------- | ||||
| Embryonal | 18 (31%) | 157 (100%) | 0 | 8 (14%) | |
| Alveolar | 35 (60%) | 0 | 83 (84%) | 41 (73%) | |
| Undifferentiated | 0 | 0 | 5 (5%) | 3 (5%) | |
| RMS, NOS | 5 (9%) | 0 | 11 (11%) | 4 (7%) | |
| Strata | -------- | ||||
| Embryonal Stage 2/3 Group III | 17 (29%) | 125 (80%) | 7 (7%) | 8 (14%) | |
| ALV/UDS Stage 1 or Group I | 18 (31%) | 0 | 18 (18%) | 5 ( 9%) | |
| ALV/UDS other | 17 (29%) | 0 | 62 (63%) | 29 (52%) | |
| PM with extension | 6 (10%) | 32 (20%) | 12 (12%) | 14 (25%) |
Distributions were compared among groups using a Chi-square test or Fisher’s exact test. No formal comparison was made for factors involving histology, which was used directly in the risk group definitions.
Prognostic Significance of Tumor Diameter and Patient Age
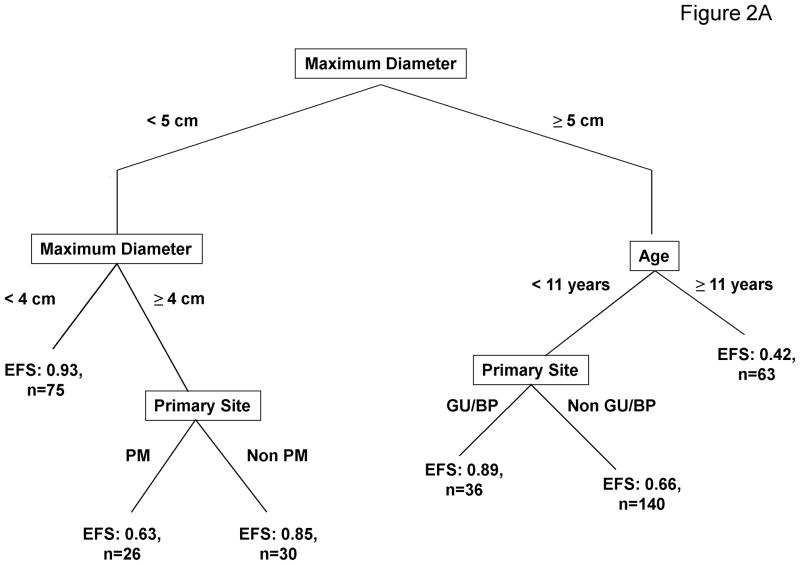
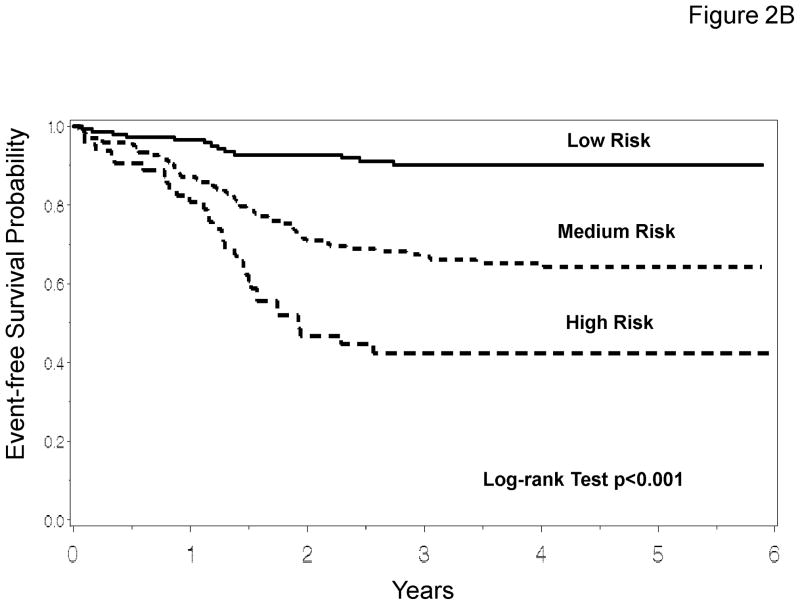
To determine if maximum tumor diameter and patient age, known predictors of outcome, were equivalent to tumor volume and patient weight for prognostic stratification, the recursive partitioning method was repeated after tumor volume, patient weight, and patient body surface area were excluded (Figure 2A). In this model the most important predictor of outcome was maximum tumor diameter. Patients with small tumors were further stratified based on tumor diameter and primary tumor site. Patients with large tumors were further stratified based on age and primary tumor site. The analysis that evaluated the traditional prognostic factors of maximum tumor diameter and patient age resulted in 6 risk groups that, based on EFS, could then be combined into three distinct risk group categories (Table 3). EFS Kaplan-Meier curves for these risk groups are presented in Figure 2B. Disease and patient characteristics for each risk group are described in Table 4. There are differences in patient age, tumor stage, tumor primary site, histology, nodal status, tumor invasiveness, and tumor size among the risk groups.
Figure 2.
Prognostic Significance of Tumor Diameter and Patient Age. 2A. Recursive partitioning algorithm utilizing the following variables as potential prognostic factors for EFS: age, stage, group, T-stage, nodal status, histology, primary site, maximum tumor diameter, patient height, and treatment regimens (tumor volume, patient weight, and body surface area were excluded). 2B. EFS Kaplan-Meier curves for risk groups identified by the recursive partitioning.
Table 3.
Risk Stratification for Event-free Survival (EFS) Based on Traditional Criteria of Maximum Tumor Diameter and Age
| Risk Group | Subject Characteristics | 4-year EFS Rate | 95% CI |
|---|---|---|---|
| Low (n=141) | Maximum Diameter < 4 cm | 0.90 | 0.84 to 0.94 |
| Maximum Diameter ≥ 4 cm and < 5 cm, Non-PM primary | |||
| Maximum Diameter ≥ 5 cm, Age < 11 years, GU/BP primary | |||
| Medium (n=166) | Maximum Diameter ≥ 4 cm and < 5 cm, PM primary | 0.65 | 0.57 to 0.72 |
| Maximum Diameter ≥ 5 cm, Age < 11 years, Non-GU/BP primary | |||
| High (n=63) | Maximum Diameter ≥ 5 cm, Age ≥ 11 years | 0.42 | 0.29 to 0.55 |
Table 4.
Patient and disease characteristics for risk stratification using traditional prognostic factors in RMS. Risk stratification groups are defined in Table 3.
| Low Risk (n=141) | Medium Risk (n=166) | High Risk (n=63) | p-value* (overall test) | |
|---|---|---|---|---|
| Count (%) | Count (%) | Count (%) | ||
| Age (years) | ---------- | |||
| <1 | 5 ( 4%) | 5 ( 3%) | ||
| 1–9 | 109 (77%) | 146 (88%) | ||
| 10+ | 27 (19%) | 15 ( 9%) | 63 (100%) | |
| Stage | <0.001 | |||
| 1 | 24 (17%) | 8 ( 5%) | 3 ( 5%) | |
| 2 | 70 (50%) | 28 (17%) | 6 (10%) | |
| 3 | 47 (33%) | 130 (78%) | 54 (86%) | |
| Group | 0.3 | |||
| I | 7 ( 5%) | 4 ( 2%) | 1 ( 2%) | |
| II | 16 (11%) | 11 ( 7%) | 5 ( 8%) | |
| III | 118 (84%) | 151 (91%) | 57 (90%) | |
| Primary Site | ---------- | |||
| Extremity | 26 (18%) | 29 (17%) | 13 (21%) | |
| GU/BP | 56 (40%) | 6 (10%) | ||
| Parameningeal | 28 (20%) | 78 (47%) | 34 (54%) | |
| Retroperitoneal/Perineal | 1 ( 1%) | 44 (27%) | 4 ( 6%) | |
| Other | 30 (21%) | 15 ( 9%) | 6 (10%) | |
| Tumor Size | ---------- | |||
| ≤ 5 cm | 103 (73%) | 34 (20%) | 9 (14%) | |
| >5 cm | 38 (27%) | 132 (80%) | 54 (86%) | |
| Tumor Invasion | <0.001 | |||
| T-1 | 85 (60%) | 65 (39%) | 22 (35%) | |
| T-2 | 56 (40%) | 101 (61%) | 41 (65%) | |
| Regional Lymph Nodes | <0.001 | |||
| N-0 | 125 (89%) | 142 (86%) | 42 (67%) | |
| N-1 | 16 (11%) | 24 (14%) | 21 (33%) | |
| Histology | <0.001 | |||
| Embryonal | 70 (50%) | 100 (60%) | 13 (21%) | |
| Alveolar | 59 (42%) | 58 (35%) | 42 (67%) | |
| Undifferentiated | 0 | 5 ( 3%) | 3 ( 5%) | |
| RMS, NOS | 12 ( 9%) | 3 ( 2%) | 5 ( 8%) | |
| Strata | <0.001 | |||
| Embryonal Stage 2/3 Group III | 70 (50%) | 74 (45%) | 13 (21%) | |
| ALV/UDS Stage 1 or Group I | 27 (19%) | 11 ( 7%) | 3 ( 5%) | |
| ALV/UDS other | 34 (24%) | 42 (25%) | 32 (51%) | |
| PM with extension | 10 ( 7%) | 39 (23%) | 15 (24%) |
Distributions were compared among groups using a Chi-square test or Fisher’s exact test. No formal comparison was made for factors involving age, maximum diameter, and primary site, which were used directly in the risk group definitions.
Comparison of Volume/Weight vs. Diameter/Age
The correlation between the risk categories utilizing the two approaches (tumor volume/patient weight vs. tumor diameter/patient age) is better for the highest and lowest risk categories, and worse for the middle risk categories. Also, there are some patients who are classified in the lowest-risk category based on tumor diameter, age, and primary site and are classified in the highest-risk category based on tumor volume, weight, and histology (Table 5). Thus although the correlation is good, it is possible that one strategy is better at predicting outcome. To determine if maximum tumor diameter and patient age were superior to tumor volume and weight to predict outcome, a Cox-proportional hazards regression model was fit with the three risk categories defined by maximum diameter, age, and primary site. Then, the risk grouping based on volume and weight was entered into the model. This methodology can determine whether the addition of volume and weight risk categories adds significant information about the risk of failure. The difference in the likelihood ratio statistic for each of these models was determined and a p-value for the loss of information was determined using a Chi-square distribution with 3 degrees of freedom. In the first model (including risk categories identified by maximum diameter, age, primary site as well as risk categories defined by tumor volume, patient weight, and tumor histology): the likelihood ratio test statistic was 63.22 with 5 degrees of freedom. For the second model (including risk categories identified by maximum diameter, age, and primary site) the likelihood ratio test statistic was only 53.36 with 2 degrees of freedom. The addition of tumor volume, patient weight, and tumor histology is important, with the difference between the likelihood ratio test statistic for the two models being 9.86 (p=0.02). Therefore, the addition of tumor volume and patient weight provides significant prognostic information to a model that includes risk categories defined by the traditional prognostic factors including tumor diameter and patient age.
Table 5.
Correlation of risk stratification based on tumor volume and patient weight vs. tumor diameter and patient age.
| Frequency Row Percent |
Grouping defined by maximum diameter, subject age, and primary site | ||
|---|---|---|---|
| Low Risk (n=141) | Medium Risk (n=166) | High Risk (n=63) | |
| Risk Group 1 (Tumor volume < 20 cm3) (n=58) | 55 95% |
3 5% |
0 |
| Risk Group 2 (Tumor volume ≥ 20 cm3, weight < 50 kg, embryonal disease) (n=157) | 52 33% |
98 62% |
7 4% |
| Risk Group 3 (Tumor volume ≥ 20 cm3, weight < 50 kg, non- embryonal disease) (n=99)) | 27 27% |
60 61% |
12 12% |
| Risk Group 4 (Tumor volume ≥ 20 cm3, weight ≥ 50 kg) (n=56) | 7 13% |
5 9% |
44 79% |
In summary, the factors most strongly associated with risk of failure are tumor volume, patient weight, and tumor histology. When volume and weight are not included as potential factors, maximum diameter, age, and primary site are most strongly associated with the risk of failure. Tumor volume and patient weight are superior predictors of outcome and may be better measures of tumor burden and patient size. In addition, patient size, relative to tumor size, is important when considering larger tumors. Among children with larger tumors, patients that are older have poorer outcomes.
Association between Tumor Size, Patient Size and Completeness of Tumor Resection and Outcome
Completeness of tumor resection, defined by clinical Group, is a component of disease risk stratification. It is possible that tumor size in relation to patient size may influence the clinical Group and therefore, outcome. Completeness of surgical resection was quantified for each patient (clinical Group I complete resection, clinical Group II microscopic residual disease, clinical Group III gross residual disease). A non-parametric Kruskal-Wallis test was utilized to evaluate the relationship between patient size and clinical Group. There was no significant difference in median age (p>0.9) nor median weight (p=0.8) among the three clinical Group categories (Table 6). This is further supported using a Cox-proportional hazard regression model that evaluated the relationship between the distribution of EFS and tumor and patient sizes after adjustment for histology, primary site, completeness of surgical resection (clinical Group), stage, nodal status, and T-stage. When considering cut-points based on median weight and tumor volume, there was no significant indication that patient weight (< 20 kg vs. ≥20 kg) significantly modified the association between tumor volume (< 90 cm3 vs. ≥90 cm3) and EFS after adjustment for standard risk factors (p=0.2). Subjects were split into 4 roughly equally sized groups defined by cut-points of 90 cm3 for tumor volume and 20 kg for subject weight. Using the large child (≥20 kg) with a small tumor (< 90 cm3) as the reference group (n=91), suggestive of lower relative tumor burden, the risk of failure is increased by 33% for a small child with a small tumor (n=92, p=0.4), increased by 63% for a small child with a large tumor (n=87, p=0.2) and increased by 112% for a large child with a large tumor (n=100, p=0.02). Even though there seems to be a trend, the difference between large subjects with small tumors (suggestive of lower relative tumor burden) and small subjects with large tumors (suggestive of higher relative tumor burden) was not statistically significant (Table 7). ). To further investigate this issue, the ratio of tumor volume (cm3) to patient weight (kg) was calculated and subjects were grouped according to quartiles of this ratio. After adjustment for histology, primary site, completeness of surgical resection (clinical Group), stage, nodal status, and T-stage, the increased hazard of failure for subjects with higher tumor volume to weight ratio values, relative to the those with tumor volume to weight ratio values < 1.25 cm3/kg (n=91), were not statistically significant (Ratio 1.25 to 3.99 cm3/kg: n=95, HR=1.71, 95% CI: 0.91 to 3.20, p=0.09; Ratio 4.00 to 9.99 cm3/kg: n=93, HR=1.92, 95% CI: 0.97 to 3.79, p=0.06; and Ratio ≥ 10 cm3/kg: n=91, HR=1.67, 95% CI: 0.73 to 3.83, p=0.2).
Table 6.
Association between Patient Size and Completeness of Resection (Surgical Group)
| Group | N | Variable | Minimum | Maximum | Median | Mean | Std. Deviation |
|---|---|---|---|---|---|---|---|
| I | 12 | Age (years) Weight (kg) |
0.91 8.10 |
17.02 92.00 |
6.08 21.60 |
7.39 33.42 |
5.41 27.30 |
| II | 32 | Age (years) Weight (kg) |
0.77 8.90 |
18.31 76.70 |
6.92 23.20 |
7.24 29.79 |
5.13 19.27 |
| III | 326 | Age (years) Weight (kg) |
0.07 3.40 |
41.86 135.00 |
5.23 20.00 |
7.22 28.64 |
5.95 21.03 |
Table 7.
Impact of Tumor Volume (split at < 90 cm3) and Patient Weight (split at < 20 kg) on Event-free Survival outcome.
| Group | HR | 95% CI | p-value |
|---|---|---|---|
| Small Tumor Small Child (n=92) |
1.33 | 0.69–2.57 | 0.4 |
| Small Tumor Large Child (n=91) |
1.00 (reference group) | ||
| Large Tumor Small Child (n=87) |
1.63 | 0.80–3.34 | 0.2 |
| Large Tumor Large Child (n=100) |
2.12 | 1.12–4.02 | 0.02 |
DISCUSSION
This study demonstrates that the factors most strongly associated with risk of failure among intermediate risk patients are tumor volume, patient weight, and tumor histology. When volume and weight are not utilized, maximum tumor diameter, age, and primary site are the factors most strongly associated with the risk of failure. While overall, tumor volume and patient weight are mathematically superior predictors of outcome, modeling based on maximum diameter and age can also be used to identify relapse risk subgroups among the intermediate-risk population. In addition, our study demonstrates that the ability to resect the tumor completely was not significantly associated with the size of the patient, and patient weight did not significantly modify the association between tumor volume and EFS after adjustment for standard risk factors.
The prognosis of patients with RMS is dependent on many factors. Favorable prognostic factors include embryonal/botryoid histology, favorable primary tumor sites, no distant metastases at diagnosis, complete gross removal of tumor at the time of diagnosis, tumor size less than or equal to 5 cm, and age less than 10 years at the time of diagnosis.6, 12–14 The extent of surgical resection (i.e., clinical Group) was identified as one of the most important predictors of treatment failure and tumor recurrence. 6, 13–15
The study by Meza et al. evaluated a total of 1,258 patients without distant metastases enrolled into either IRS III or IRS IV between 1984 and 1997.3 In this study, prognostic factors for alveolar RMS included tumor stage and clinical Group, age less than one year and local regional lymph node disease. For patients with embryonal RMS, both stage and clinical Group were significantly associated with failure-free survival (FFS). Other predictive factors included age less than one year old or greater than 10 years old, unfavorable site of primary tumor, tumor size >5cm and tumor invasiveness. The FFS for the patients ranged from 90% for patients with embryonal RMS with stage 1, clinical Group I disease down to 45% for patients with alveolar RMS at unfavorable sites that were clinical Group III. This broad range of FFS demonstrated the importance of prognostic factors to allow allocation of patients to the most appropriate intensity of therapy. In this current study intermediate risk RMS patients from D9803 were analyzed to compare traditional prognostic factors with the potential prognostic factors tumor volume and patient weight. Our results support the conclusion that tumor volume and patient weight are important prognostic markers and may be more predictive than the traditional factors of tumor diameter and patient age. These results are different than those of Ferrari et al.8 This single institution retrospective study of 205 patients from 1982 − 2008 demonstrated that tumor diameter and volume were equally predictive of survival
It has been postulated that the size of the tumor relative to the size of the patient may have an impact on the outcomes for children with RMS. Ferrari et al. showed that for non-RMS STS, increased tumor size was stronger unfavorable prognostic factor in children than in adults.16 In a subsequent study they evaluated 553 patients less than 21 years old with non-metastatic STS treated between 1977 and 2005.7 The 5 and 10 year overall survival estimates among the 304 subjects with RMS were 64% and 63% respectively. Tumor diameter was statistically significant as a prognostic marker of overall survival and the interaction between tumor size and body surface area was significant for RMS patients (p=0.03), but not other STS patients. Increasing tumor diameter was an unfavorable prognostic factor regardless of body surface area, but particularly for subjects with small body surface area. They also demonstrated that in tumors < 2 cm, the effect of body surface area and mortality tended to be diminished. This is supported by our study in which subjects with tumors of small volume have very good EFS, regardless of subject weight. However, our study demonstrates no significant differences in the age or weight distribution among the clinical Group categories suggesting that patient weight did not impact successful tumor resection. While large children with large tumors had significantly poorer prognosis relative to large children with small tumors, there was no significant indication that the outcomes were worse for small subjects with large tumors compared to large subjects with small tumors.
The differences in observations between the results of Ferrari et al.7, 8 and our study may reside in the different treatment paradigms utilized by European and North American studies specifically regarding the use of radiotherapy. It is possible that the more aggressive local control philosophy used in COG studies minimizes the impact of tumor size in relation to patient size. In addition, the study by Ferrari included all patients with non-metastatic RMS (low and intermediate risk patients) whereas our study only evaluated intermediate risk patients. Another possible explanation is that weight may be a more reliable prognostic indicator as compared with BSA when assessing the contribution of body proportion to outcome. In our analysis patient weight, but not body surface area, was selected as an important prognostic factor thus indicating that weight may be a better indicator of patient size. In addition, our observations hold true regardless of whether tumor volume or diameter were used as the indicator of tumor size.
The current study has several limitations. Tumor dimensions (maximal diameter and volume) were submitted by institutions after local review of imaging studies. Variations in the techniques used for tumor measurement may have led to inaccurate reporting of tumor size. Tumor volume was missing for 33% of the D9803 study participants. The subjects analyzed in this report differed significantly from the subjects missing tumor volume measurements or other clinical data. These differences may have had an uncontrolled impact on our current results and conclusions. Finally, our analysis included only intermediate risk patients and our findings cannot be extended to either low or high risk patients without further evaluation. Validation of these results will be required to extend the observations to all patients with RMS.
In conclusion, tumor volume and patient weight are strongly associated with risk of failure and may have better predictive value than traditional prognostic factors, may improve disease risk stratification, and subsequently allow therapy to be more accurately tailored to disease risk. These findings require confirmation in low- and high-risk patients to be broadly applicable to all RMS patients.
Acknowledgments
Support: Research is supported by the U10 CA24507 (Intergroup Rhabdomyosarcoma Study Group) Statistics and Data Center Grant U10 CA98413, and the U10 CA98543 (Children’s Oncology Group Chair’s Grant) from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Footnotes
Presented at the American Pediatric Surgical Association 2008
Disclaimers: None
References
- 1.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 2.Hayes-Jordan A, Andrassy R. Rhabdomyosarcoma in children. Curr Opin Pediatr. 2009;21(3):373–378. doi: 10.1097/MOP.0b013e32832b4171. [DOI] [PubMed] [Google Scholar]
- 3.Meza JL, Anderson J, Pappo AS, Meyer WH. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on Intergroup Rhabdomyosarcoma Studies III and IV: The Children's Oncology Group. J Clin Oncol. 2006;24(24):3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 4.Stevens MC, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology - SIOP. Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23(12):2618–2628. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 5.Raney RB, Anderson JR, Barr FG, et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup Rhabdomyosarcoma Study Group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 2001;23(4):215–220. doi: 10.1097/00043426-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Joshi D, Anderson JR, Paidas C, Breneman J, Parham DM, Crist W. Age is an independent prognostic factor in rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer. 2004;42(1):64–73. doi: 10.1002/pbc.10441. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari A, Miceli R, Meazza C, et al. Soft tissue sarcomas of childhood and adolescence: the prognostic role of tumor size in relation to patient body size. J Clin Oncol. 2009;27(3):371–376. doi: 10.1200/JCO.2007.15.4542. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari A, Miceli R, Meazza C, et al. Comparison of the prognostic value of assessing tumor diameter versus tumor volume at diagnosis or in response to initial chemotherapy in rhabdomyosarcoma. J Clin Oncol. 2010;1028(8):1322–1328. doi: 10.1200/JCO.2009.25.0803. [DOI] [PubMed] [Google Scholar]
- 9.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group Study D9803. J Clin Oncol. 2009;1;27(31):5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group Data Base. J Clin Oncol. 1990;8:1563–1574. doi: 10.1200/JCO.1990.8.9.1563. [DOI] [PubMed] [Google Scholar]
- 11.Segal MR. Regression trees for censored data. Biometrics. 1988;44:35–47. [Google Scholar]
- 12.Kwak LW, Jalpern J, Olshen RA, Horning SJ. Prognostic significance of actual dose intensity in diffuse large–cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–977. doi: 10.1200/JCO.1990.8.6.963. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence W, Jr, Gehan EA, Hays DM, Beltangady M, Maurer HM. Prognostic significance of staging factors of the UICC staging system in childhood rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS-II) J Clin Oncol. 1987;1:46–54. doi: 10.1200/JCO.1987.5.1.46. [DOI] [PubMed] [Google Scholar]
- 14.Reboul-Marty J, Quintana E, Mosseri V, et al. Prognostic factors of alveolar rhabdomyosarcoma in childhood. An International Society of Pediatric Oncology study. Cancer. 1991;68(3):493–498. doi: 10.1002/1097-0142(19910801)68:3<493::aid-cncr2820680308>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Dantonello TM, Int-Veen C, Winkler P, et al. Initial patient characteristics can predict pattern and risk of relapse in localized rhabdomyosarcoma. J Clin Oncol. 2008;26(3):406–413. doi: 10.1200/JCO.2007.12.2382. Erratum in: J Clin Oncol. 2008;26(11)1911. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari A, Miceli R, Casanova M, et al. Adult-type soft tissue sarcomas in paediatric age: a nomogram-based prognostic comparison with adult sarcoma. Eur J Cancer. 2007;43(18):2691–2697. doi: 10.1016/j.ejca.2007.09.012. [DOI] [PubMed] [Google Scholar]