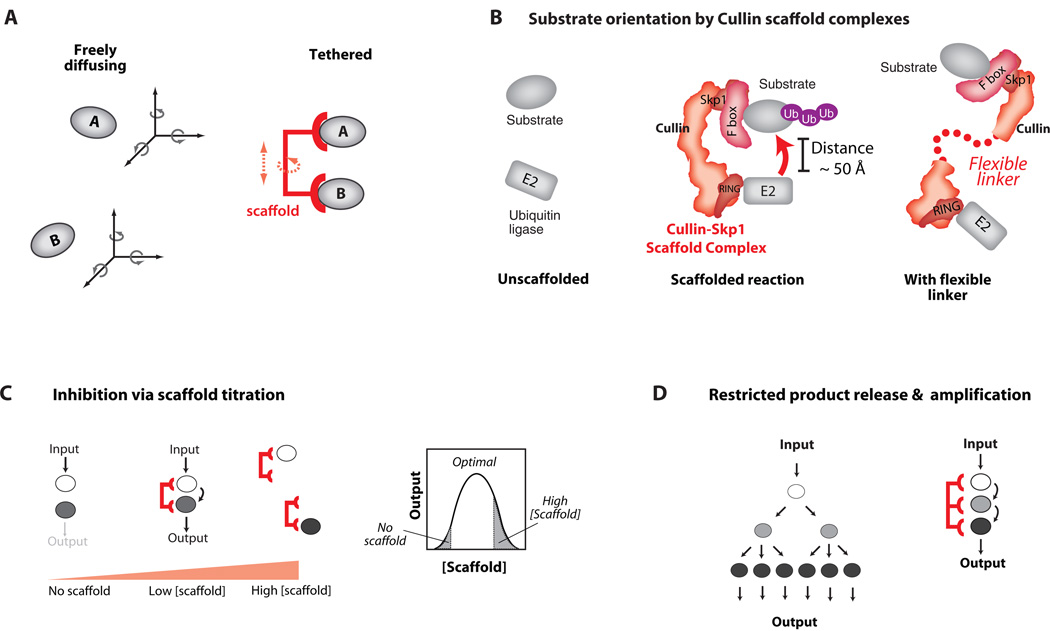
Fig. 3. Benefits and Costs of Scaffold Tethering Mechanisms.
A) By co-localizing enzyme and substrate, scaffold proteins can lower the entropic cost of signaling interactions – the loss of independent translational and rotational degrees of freedom is paid through binding interactions with the scaffold. The size of the advantage gained depends on the flexibility of the scaffold structure. B) By restricting the conformational freedom of interacting proteins, scaffolds can orient these molecules to enhance the rate of signal transfer. The rigid Cullin scaffold proteins tether E2 ubiquitin conjugating enzymes and their substrates. If the Cullin backbone is made flexible by mutation, the rate of substrate ubiquitination is greatly decreased. C) Tethering has potential drawbacks: at high concentrations scaffolds may titrate enzyme and substrate away from one another. D) Increased affinities can restrict substrate release and diffusion throughout the cell, potentially limiting signal amplification and spatial redistribution (e.g. nuclear localization).