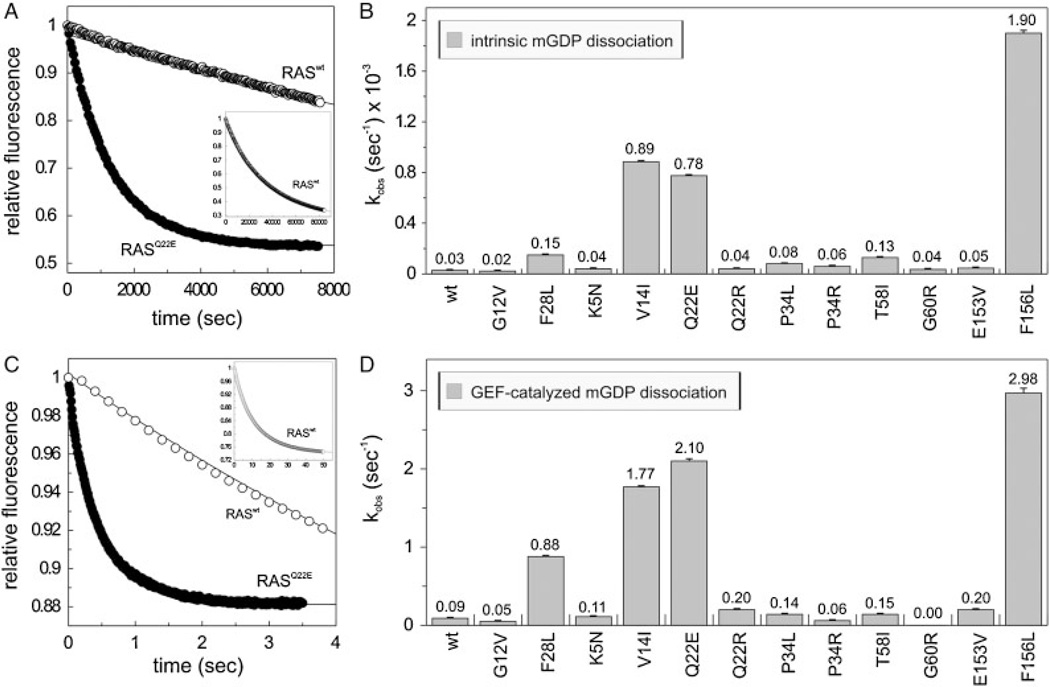
Figure 3.
Modified nucleotide exchange properties of the RAS mutants. Intrinsic (A, B) and GEF-catalyzed (C, D) mantGDP dissociation from the RAS proteins (0.2 µM) in the presence of 40 µM GDP (A) or of 40 µM GDP and 2 µM CDC25 (C). On the panels A and C the respective time-dependent reactions of RASwt and a representative RAS mutant (p.Q22E) are shown. On the panels B and D the observed rate constant (kobs) of all RAS proteins are illustrated. RASwt, RASG12V, and RASF28L were included as controls. The insets (in A and C) show the complete time course of the mantGDP dissociation from RASwt. Standard errors of five to seven independent measurements are shown.