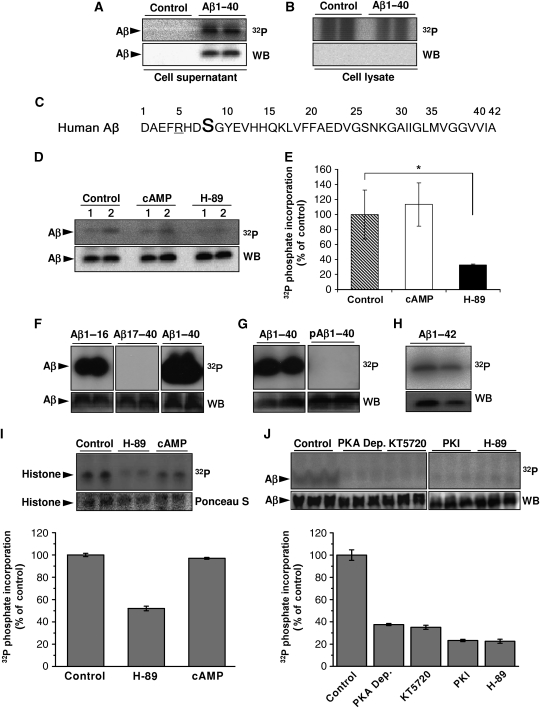
Figure 1.
Phosphorylation of Aβ at Ser8. (A, B) Primary cultures of mouse cortical neurons were incubated with 10 μM [γ-32P]ATP in the presence or absence of synthetic Aβ1-40 for 30 min at 37°C. After incubation, Aβ was immunoprecipitated from cell supernatants (A) and cell lysates (B) and was separated by SDS–PAGE, transferred onto nitrocellulose membranes and detected by autoradiography (32P) and WB. (C) Amino-acid sequence of human Aβ in single letter code. Ser8 (bold) and arginine residue 5 (underlined) resemble a consensus motif for PKA. (D, E) Phosphorylation of Aβ1-40 by primary mouse cortical neurons (1, 1 × 106 cells; 2, 2 × 106 cells) in the absence or presence of 2.5 μM cAMP or 1 μM H-89 were performed as described above and quantified by phospho-imaging (E). 32P-values represent means±s.d. (n=3). Statistical significance was evaluated by paired t-test (*P<0.05). (F–H) In vitro phosphorylation of Aβ variants by purified PKA. Synthetic peptides representing Aβ1-16, Aβ17-40, Aβ1-40 (F), Aβ1-40 phosphorylated at Ser8 (pAβ1-40; (G)) and Aβ1-42 (H) were incubated with purified PKA and [γ-32P]ATP for 15 min at 32°C. pAβ variants were detected by autoradiography followed by WB with appropriate antibodies (Aβ1-16, Aβ1-40, Aβ1-42, pAβ1-40 with antibody 82E1; Aβ17-40 with antibody 4G8). (I, J) Ex vivo phosphorylation in human CSF. Human CSF was incubated with 10 μM [γ-32P]ATP together with histone (I) or Aβ1-40 (J) in the absence or presence of the indicated kinase inhibitors (H-89, PKI and KT5720) or cAMP. PKA Dep., CSF after immunodepletion of PKA with specific antibodies. The phosphate incorporation was detected by autoradiography (32P) and quantified by phospho-imaging. Phosphorylation of Aβ was also detected with lower concentrations of ATP (1.0 and 0.1 μM; data not shown). Histone and Aβ was visualized by staining with Ponceau S and WB, respectively. Values represent means±s.d. of three independent experiments.