Abstract
Objective
This report compares the accuracy of three prediction models for the development of primary open-angle glaucoma (POAG). The models differ primarily in their handling of these eye-specific variables: intraocular pressure (IOP), central corneal thickness (CCT), vertical cup-to-disc ratio (VCD) and visual field pattern standard deviation (PSD). 1). The “means” model includes age and the means of right and left eyes, 2). The “means plus asymmetry” model includes age, the means of right and left eyes as well as the absolute difference between eyes for eye-specific variables, 3). The “worse” eye model includes age and values from the eye at higher risk for developing POAG.
Design
This report uses data from the observation group of the Ocular Hypertension Treatment Study (OHTS) and the placebo group of the European Glaucoma Prevention Study (EGPS) who have complete data on both eyes at baseline. Performance of the prediction models is assessed using the c-statistic, calibration chi-square and Pearson correlation coefficient.
Participants
The OHTS observation group (n=717, 6.7 years median follow-up) and the EGPS placebo group (n=324, 4.9 years median follow-up).
Testing
Baseline data included demographic characteristics, medical history, ocular examination, visual fields and optic disc photographs.
Main Outcome Measures
Development of reproducible visual field abnormality or optic disc deterioration as determined by masked readers and attributed to POAG by a masked endpoint committee.
Results
Baseline factors that were statistically significant in all predictive models were-age, IOP, CCT, VCD and PSD. Also, statistically significant were baseline asymmetry in IOP and asymmetry in VCD. The c-statistics for the “means” model, “means plus asymmetry” model and “worse” eye model were 0.74, 0.77 and 0.75 respectively. The calibration chi-squares were 7.32, 11.19 and 1.81 respectively. Correlation coefficients between risk estimates calculated by different models ranged from 0.94 to 0.98.
Conclusions
The high agreement between the risk estimates from three different predictive models for the development of POAG suggests little difference in their statistical or clinical performance. The predictive model that uses the means of both eyes for eye-specific variables is the simplest to use and the most robust to measurement variability and error.
Introduction
The Ocular Hypertension Treatment Study (OHTS) published a multivariate prediction model to calculate the 5-year risk for developing primary open-angle glaucoma (POAG) in ocular hypertensive individuals. Factors used to calculate risk in this prediction model included older age and several baseline eye-specific measures including vertical cup-to-disc ratio (VCD), intraocular pressure (IOP), pattern standard deviation (PSD), and central corneal thickness (CCT).1 This prediction model has been replicated in an independent U.S. study2 as well as in the European Glaucoma Prevention Study (EGPS)3 and further refined using the pooled OHTS and EGPS samples.4 The OHTS/EGPS prediction model has demonstrated good predictive accuracy across the range of risk among participants in these studies.4
The OHTS/EGPS prediction model uses the means of the right and left eyes of each participant to calculate eye-specific predictive factors. Advantages of using the means of right and left eyes include the use of all relevant data from both eyes of each participant and the reduction of measurement variability. The disadvantages of using the means of both eyes include the loss of predictive accuracy from information unique to each eye and from the differences between the eyes. This report assesses whether the accuracy and clinical application of the OHTS/EGPS predictive model based on the means of right and left eyes, “means model,” as described above is equal, superior or inferior to predictive models that includes eye-specific information: 1) a “means plus asymmetry” model, which includes the means of both eyes as well as the absolute differences between right and left eyes for eye-specific variables. 2) a “worse” eye model which includes baseline information only from the eye at higher risk of developing POAG.4
Methods
The OHTS5 and the EGPS6 are both randomized clinical trials that tested the safety and efficacy of topical ocular hypotensive medication in delaying or preventing the development of POAG in individuals with ocular hypertension. The OHTS and the EGPS protocols are described in their respective baseline design papers.7,8 The OHTS protocol is also available on the web at https://vrcc.wustl.edu (date accessed: 8/1/2006). The protocol is compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 and was approved by the institutional review boards of all participating clinics and resource centers. The OHTS registration number is NCT00000125 and can be found at www.clinicaltrials.gov (accessed March 18, 2008).
In the OHTS and the EGPS, participants were randomized in equal proportions to a control group or to a medication group. In the OHTS, the control group was an observation group that received no ocular hypotensive medication or placebo. In the EGPS, the control group was a placebo group, which received the diluent for the medication. This report uses data from participants in the OHTS and the EGPS who were randomized to the control group and did not receive active ocular hypotensive treatment. Data from these control groups provide information on the relationship of baseline factors to the true, natural (untreated) history of the risk of developing POAG.
The analysis dataset for this report includes baseline and POAG outcome data for OHTS observation participants from the start of randomization in February 1994 to June 2002, and EGPS placebo group participants from the start of randomization in January 1997 to May 2004. Only participants with complete baseline data in both eyes were included in this report so that the same sample of participants would be used to compare the performance of the different predictive models (OHTS n=717 and EGPS n=324). In the section that follows, we briefly describe the protocol for the measurement of eye-specific factors and POAG endpoints for OHTS and EGPS. Procedures for the resolution of differences between the OHTS and EGPS protocols are described in detail in a previous publication.4
IOP measurements by Goldmann tonometry. In the OHTS, the mean IOP for each eye was calculated using 2 to 3 IOP measurements from each of the two qualifying visits and the randomization visit. Thus, the mean pressure for each eye was calculated from 6 to 9 IOP measurements and the two means were averaged to create a new baseline IOP. In the EGPS, the mean IOP for each eye was calculated using 2 to 3 measurements per eye at the eligibility visit and one measurement per eye at the six-month follow-up visit. Thus, the pressure for each eye was calculated from 3 to 4 IOP measurements and the means for the two eyes were averaged.
CCT measurements were performed using the same protocol and same model pachymeter (DGH Pachette Model 500). The CCT for each eye was the mean of five measurements taken of each eye completed on a single visit and the means for the two eyes were averaged.
Optic disc: VCD was estimated by masked readers from stereoscopic slides taken by certified study photographers.
Visual fields: In the OHTS, all visual fields were assessed using full threshold white on white Humphrey standard program 30-2. In the EGPS, visual fields were assessed using Humphrey 30-2 visual fields for 80% of the participants and Octopus 32 visual fields for 20% of the participants. We converted the baseline Octopus mean defect to Humphrey mean deviation by changing the sign and the loss variance to PSD by taking the square root of the loss variance.9 The baseline visual field score for each eye is the mean of 2–3 visual fields completed at 2–3 visits and the means for the two eyes were averaged.
In both the OHTS and the EGPS, the date of onset for POAG is the date of the first of three consecutive abnormal visual fields or the first optic disc photograph that masked readers classify as meeting the definition for change and that was subsequently attributed to POAG by a masked endpoint committee. In OHTS, a technically acceptable visual field was considered abnormal if p < 5% for the corrected pattern standard deviation or if the glaucoma hemifield test was outside normal limits by STATPAC 2.7 In EGPS, a visual field was considered abnormal if three or more adjacent points were reduced by ≥ 5 dB from baseline, or were reduced by two or more adjacent points differ ≥ 10 dB from baseline.8
Baseline demographic and clinical information in both the OHTS and EGPS was collected on each participant prior to randomization, except for CCT measurements, which were performed 1–3 years after randomization.
Statistical Analysis
The prediction models for the development of POAG differ in their handling of eye-specific measures (IOP, CCT, VCD and PSD). Eye-specific variables in the “means” model were the means of right and the left eyes of each participant. Eye-specific variables in the “means plus asymmetry” model were the means of right and left eyes and their absolute differences for each participant.
Eye-specific variables in the “worse” eye model first required that we identify which eye is “worse” based on four eye-specific factors: IOP, CCT, VCD and PSD. No one factor captured the risk status of an eye. The risk of each eye was calculated using the OHTS/EGPS multivariate prediction model and the eye with the higher risk was selected as the “worse” eye. The 5-year risk of developing POAG of each eye could range from 0.00 to 1.00 or 0% to 100%. If both eyes had equal (2 decimal places) risk of developing POAG, one eye was selected randomly. Because the OHTS/EGPS multivariate model, which was used to calculate the risk for the worse eye, was developed from this same sample of participants, statistical circularity is introduced. This statistical circularity could increase the apparent accuracy of the “worse” eye model.
Multivariate Cox proportional hazards models were used to calculate hazard ratios for baseline factors predictive for the development of POAG for each of the different models, the “means” model, the “means plus asymmetry” model and the “worse eye” model. The accuracy of each prediction model in discriminating between participants who did or did not develop POAG was assessed using the c-statistic.10 The c-statistic ranges from 0.50 (chance) to 1.00 (perfect agreement). The rate of over/under estimation of the actual number of POAG events compared to the observed number of POAG events was calculated for each prediction model using the calibration chi-square.11 The calibration chi-square divides the sample into 10 levels of risk; for each decile, the predicted risk of developing POAG is compared to the observed proportion of participants developing POAG. A calibration chi-square of 20.00 and below indicates good agreement between the predicted and the observed event rate.11
The agreement between the risk estimates calculated for each participant by the three predictive models was assessed using Pearson correlation coefficients and Spearman rank order correlation coefficients.
The clinical application of each model was assessed by examining the stability of the risk estimates. In both OHTS and EGPS an emphasis was placed on measurement reliability. For instance, two to nine measurements of IOP were performed per eye to get a stable estimate of baseline IOP. However, one IOP measurement per eye is typically performed in clinical practice. Thus, to investigate the stability of the “means plus asymmetry” model in a clinical setting, we calculated baseline IOP and asymmetry of IOP using only the first of 2–9 IOP measurements per eye. We compared the results of the “means plus asymmetry” model based on 2–9 IOP measurements per eye to the same model based on one IOP measurement per eye. A similar analysis with cup-to-disc ratio could not be conducted because only one grading of cup-to-disc ratio is available at baseline in OHTS and in EGPS.
The clinical application of the “worse” eye model was assessed by examining whether the same eye was selected the “worse” eye at baseline and at 12 months. We calculated the risk estimate for right and left eyes using data at baseline and recalculated the risk estimate again using data at 12 months. We determined whether the same eye was designated the “worse” eye at both the baseline and at the 12-month follow-up visit. This analysis was conducted using data from the OHTS observation group because all data on predictive factors were available for both eyes of all participants at both baseline and 12 months.
Results
Baseline demographic and clinical features of participants who did or did not develop POAG in the OHTS observation group and the EGPS placebo group are reported in Table 1.
Table 1.
Comparison of baseline risk factors for participants who did and did not develop primary open-angle glaucoma (POAG)
| Develop POAG | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | ||||||||
| N | Mean | Std | N | Mean | Std | N | Mean | Std | |
| Age | 898 | 55.8 | 9.7 | 143 | 59.18 | 9.2 | 1041 | 56.22 | 9.7 |
| Mean OU Intraocular Pressure (IOP) | 898 | 24.2 | 2.3 | 143 | 25.14 | 2.7 | 1041 | 24.33 | 2.4 |
| Mean OU Central Corneal Thickness (CCT) | 898 | 577.6 | 36.3 | 143 | 552.1 | 35.8 | 1041 | 574.1 | 37.3 |
| Mean OU Vertical Cup-to-Disc Ratio | 898 | 0.4 | 0.2 | 143 | 0.45 | 0.2 | 1041 | 0.37 | 0.2 |
| Mean OU Pattern Standard Deviation (PSD) | 898 | 1.93 | 0.4 | 143 | 2.02 | 0.3 | 1041 | 1.94 | 0.4 |
| Absolute difference between eyes-IOP | 898 | 1.33 | 1.3 | 143 | 1.93 | 1.7 | 1041 | 1.41 | |
| Absolute difference between eyes-CCT | 897 | 8.69 | 7.6 | 142 | 8.57 | 7.9 | 1039 | 8.67 | 7.7 |
| Absolute difference between eyes-Vertical Cup-to-Disc Ratio | 898 | 0.07 | 0.1 | 143 | 0.11 | 0.1 | 1041 | 0.08 | 0.1 |
| Absolute difference between eyes-Pattern Standard Deviation | 897 | 0.25 | 0.3 | 141 | 0.28 | 0.2 | 1038 | 0.25 | 0.3 |
| Worse eye IOP | 898 | 24.6 | 2.5 | 143 | 25.71 | 3.0 | 1041 | 24.71 | 2.6 |
| Worse eye CCT | 898 | 575.4 | 36.8 | 143 | 550.2 | 36.8 | 1041 | 572.0 | 37.8 |
| Worse eye Vertical Cup-to-Disc Ratio | 898 | 0.38 | 0.2 | 143 | 0.49 | 0.2 | 1041 | 0.39 | 0.2 |
| Worse eye PSD | 898 | 2.00 | 0.5 | 143 | 2.10 | 0.4 | 1041 | 2.02 | 0.5 |
Std = Standard deviation
OU = Right and left eyes
“Means” Prediction Model
In the “means” prediction model, which used the means of right and left eyes for eye-specific variables, statistically significant baseline predictors for the development of POAG in the multivariate Cox proportional hazards model included baseline age, mean IOP, mean CCT, mean VCD and mean PSD (Table 3).
Table 3.
Hazard ratios and 95% confidence intervals for baseline predictors for the development of Primary Open-Angle Glaucoma (POAG) in the “means” model, the “means plus asymmetry” model and the “worse eye” model
| Hazard ratio and 95% confidence interval (CI) for… | 5 variable model (n=1041) |
5 variable model + 4 asymmetry variables (n=1036) |
5 variable model + asymmetry in IOP and C/D ratio (n=1041) |
“Worse” eye model |
|---|---|---|---|---|
| Age decade | 1.27 (1.07, 1.51) | 1.27 (1.05, 1.52) | 1.27 (1.06, 1.52) | 1.29 (1.09, 1.54) |
| Intraocular Pressure (IOP) | 1.11 (1.04, 1.19) | 1.11 (1.03, 1.19) | 1.10 (1.03, 1.18) | 1.12 (1.05, 1.19) |
| Mean Central Corneal Thickness (CCT) per 40 micron decrease | 1.98 (1.64, 2.40) | 1.88 (1.55, 2.28) | 1.91 (1.58, 2.31) | 1.92 (1.59, 2.31) |
| Vertical Cup-to-Disc ratio per 0.1 | 1.22 (1.11, 1.34) | 1.25 (1.13, 1.38) | 1.24 (1.12, 1.37) | 1.27 (1.16, 1.39) |
| Pattern Standard deviation (PSD) per 0.2 | 1.17 (1.06, 1.29) | 1.14 (1.01, 1.29) | 1.18 (1.07, 1.31) | 1.13 (1.07, 1.20) |
| Absolute difference IOP | 1.12 (1.02, 1.24) | 1.13 (1.03, 1.24) | ||
| Absolute difference CCT per 40 micron decrease | 1.19 (0.49, 2.86) | n/a | ||
| Absolute difference Vertical Cup-to-Disc per 0.1 | 1.50(1.26, 1.79) | 1.48(1.25, 1.76) | ||
| Absolute difference PSD per 0.2 | 1.06 (0.97, 1.16) | n/a | ||
| C-statistic with 95% CI | 0.74 (0.70, 0.79) | 0.77 (0.72, 0.81) | 0.77 (0.72, 0.81) | 0.75 (0.71, 0.80) |
| Calibration Chi-Square | 7.32 | 9.17 | 11.19 | 1. 81 |
+ IOP is the mean of the initial IOP per eye for the participant at the baseline visit.
“Means plus Asymmetry” Prediction Model
The range and magnitude of asymmetry between eyes in IOP, CCT, VCD and PSD were modest (Table 1 and 2). Twelve percent (121 of 1,041) of the participants had an IOP difference between eyes at baseline of greater than 3 mm Hg (Table 2). Six percent (64 of 1,041) of the participants had a difference between eyes at baseline of greater than 0.2 in VCD.
Table 2.
Distribution of absolute differences between right and left eyes for Intraocular Pressure (IOP), Central Corneal Thickness (CCT), vertical cup-to-disc ratio, Pattern Standard Deviation (PSD) and the development of Primary Open-Angle Glaucoma (POAG) in one or both eyes
| N | % | At POAG endpoint in one or both eyes? | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| N | % | N | % | |||
| Absolute difference in IOP | 793 | 76.2 | 701 | 88.4 | 92 | 11.6 |
| <= 2 | ||||||
| >2 thru 3 | 127 | 12.2 | 109 | 85.8 | 18 | 14.2 |
| >3 thru 4 | 69 | 6.6 | 53 | 76.8 | 16 | 23.2 |
| >4 | 52 | 5.0 | 35 | 67.3 | 17 | 32.7 |
| Absolute difference in CCT | 699 | 67.3 | 607 | 86.8 | 92 | 13.2 |
| <= 10 | ||||||
| >10 thru 15 | 172 | 16.6 | 146 | 84.9 | 26 | 15.1 |
| >15 thru 20 | 80 | 7.7 | 66 | 82.5 | 14 | 17.5 |
| >20 | 88 | 8.5 | 78 | 88.6 | 10 | 11.4 |
| Absolute difference in vertical cup-to-disc ratio | 732 | 70.3 | 652 | 89.1 | 80 | 10.9 |
| <= 0.1 | ||||||
| >0.1 thru 0.2 | 245 | 23.5 | 197 | 80.4 | 48 | 19.6 |
| >0.2 thru 0.3 | 42 | 4.0 | 35 | 83.3 | 7 | 16.7 |
| >0.3 | 22 | 2.1 | 14 | 63.6 | 8 | 36.4 |
| Absolute difference in PSD | 1034 | 99.6 | 893 | 86.3 | 141 | 13.6 |
| <= 2 | ||||||
| >2 thru 3 | 2 | 0.2 | 2 | 100.0 | 0 | 0 |
| >4 | 2 | 0.2 | 2 | 100.0 | 0 | 0 |
In the “means plus asymmetry” prediction model, statistically significant baseline predictors for the development of POAG in the multivariate Cox proportional hazards model included baseline age, mean IOP, mean CCT, mean VCD, mean PSD, the absolute difference between eyes in IOP, and the absolute difference between eyes in VCD (Table 3). The absolute differences between eyes in CCT and PSD were not statistically significant in the prediction model.
When the “means plus asymmetry” model was recalculated using only the first of 2–9 IOP measurements per eye to mimic clinical practice, baseline IOP remained statistically significant in univariate and multivariate Cox proportional hazards models. However, the absolute difference in IOP between eyes was no longer statistically significant (hazard ratio of 0.99; 95% confidence limits 0.90–1.10, p=0.94) and was not selected for inclusion in the multivariate model (Table 3). A parallel analysis on VCD could not be performed because only one assessment of VCD was available at baseline.
“Worse Eye” Prediction Model
The selection of the “worse” eye proved difficult because the eyes of many participants had nearly identical risk estimates at baseline. Nineteen percent (194 of 1,041) of the participants had the same risk score in both eyes to two decimal places. Of the 847 participants with an eye that could be defined as “worse,” 90% (763 of 847) had a difference in risk between eyes of less than 0.10, e.g., a 5-year risk of developing POAG of 5% in one eye versus 15% in the fellow eye. Only 2 percent (17 of 847) of the participants had a difference in risk between eyes of 0.20 or greater.
POAG developed in 11.0% (94 eyes of 847 participants) of the “worse” eyes at baseline, in 6.0% (51 eyes of 847 participants) of the “better” eyes and in 8.2% (16 eyes of 194 participants) of the eyes that had the same risk estimate.
In the “worse eye” prediction model, statistically significant baseline predictors for the development of POAG in the multivariate Cox proportional hazards model included baseline age, IOP, CCT, VCD and PSD for the worse eye as defined by the risk model (Table 3).
The stability of the “worse” eye model was assessed in the OHTS observation group by comparing whether the same eye defined as “worse” at baseline continued to be the “worse” eye at 12 months. Of the 649 OHTS observation participants, 81% (524 of 649) had an eye that could be designated the “worse” eye. In these 524 eyes that were defined as the “worse” eye at baseline, 22% (116 of 524) were defined as the “better” eye at 12 months and 10% (55 of 524) were identical in risk (to two decimal places) as the fellow eye at 12 months.
Comparison of Prediction Models for POAG
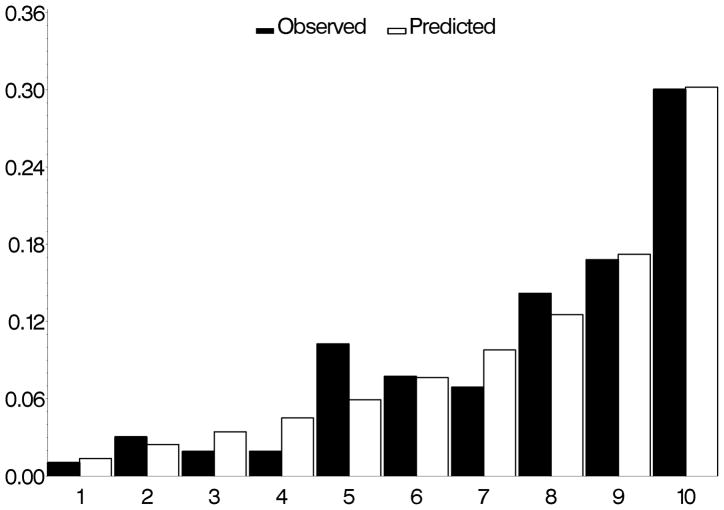
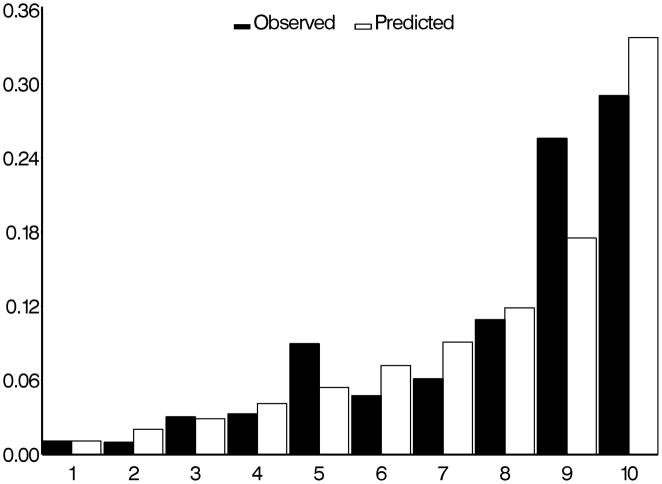
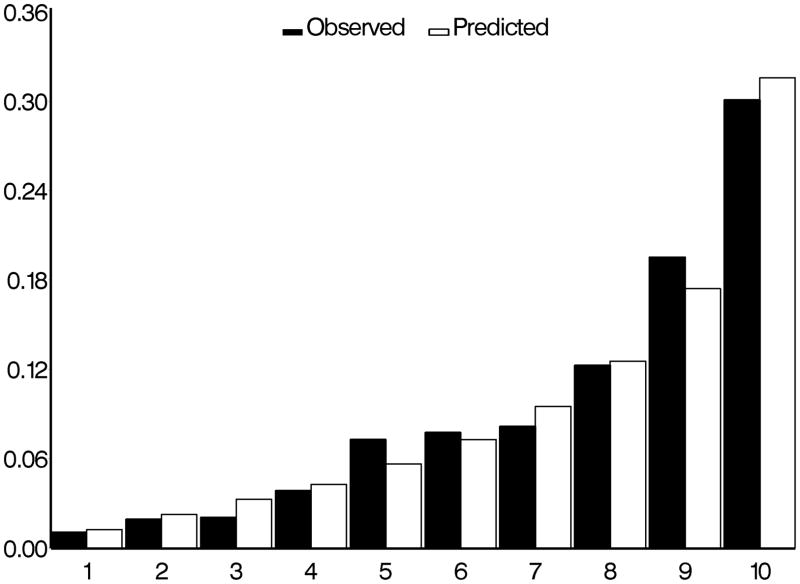
The c-statistics for concordance between observed and predicted development of POAG for the “means” model, the “means plus asymmetry” model and “worse eye” model were 0.74, 0.77, and 0.75 respectively (higher values indicate better concordance). The calibration chi-squares for “means” model, the “means plus asymmetry” model and “worse eye” model were 7.32, 11.19 and 1.81 respectively where lower values indicate less over/under estimation (Figures 1, 2 and 3).
Figure 1.
Comparison of observed and predicted five-year incidence of POAG for the OHTS-EGPS pooled “mean” model. The x-axis refers to the predicted probability of risk divided into 10 groups of approximately 90 participants each.
POAG=Primary open-angle glaucoma; OHTS=Ocular Hypertension Treatment Study; EGPS=European Glaucoma Prevention Study
Figure 2.
Comparison of observed and predicted five-year incidence of POAG for the OHTS-EGPS pooled “asymmetry” model. The x-axis refers to the predicted probability of risk divided into 10 groups of approximately 90 participants each.
POAG=Primary open-angle glaucoma; OHTS=Ocular Hypertension Treatment Study; EGPS=European Glaucoma Prevention Study
Figure 3.
Comparison of observed and predicted five-year incidence of POAG for the OHTS-EGPS pooled “worse eye” model. The x-axis refers to the predicted probability of risk divided into 10 groups of approximately 90 participants each.
POAG=Primary open-angle glaucoma; OHTS=Ocular Hypertension Treatment Study; EGPS=European Glaucoma Prevention Study
The Pearson correlation coefficient for the risk of developing POAG calculated by each model for each participant were high, ranging from 0.94 to 0.98 and the Spearman rank order correlation coefficient had the identical range.
Discussion
The OHTS/EGPS collaborative prediction model uses five baseline factors: age, IOP, CCT, VCD and PSD to predict the 5-year risk of developing POAG. All eye-specific factors in this model were calculated as the means of right and left eyes and thus, information about differences between eyes was not used.4 We undertook this re-analysis to determine if the predictive accuracy of the OHTS/EGPS prediction model could be improved by incorporating eye-specific information. The prognostic value of asymmetry between right and left eyes for the development of POAG in ocular hypertension has been reported for IOP 12, cup-to disc ratio3,13 and visual field threshold.12 In addition, greater asymmetry between eyes of established glaucoma patients compared to controls has been reported for many ocular measures including disc parameters14–18, IOP19, contrast sensitivity20, visual evoked response21 and blood velocity22. These studies suggest that asymmetry between eyes may be a sentinel for incipient glaucoma and/or a marker for established glaucoma.
We found that the performance of the OHTS/EGPS prediction model based on the means of right and left eyes for eye-specific variables was remarkably comparable to prediction models that included additional information on baseline differences between eyes. The correlation coefficients for risk scores of each participant calculated from the “means,” “means plus asymmetry” and “worse” eye models ranged between 0.94 and 0.98 and were statistically equivalent. The three predictive models for the development of POAG identified the same five baseline risk factors: age, IOP, CCT, vertical cup disc-to-ratio and PSD. In addition, the “means plus asymmetry” model identified larger baseline asymmetry in IOP and larger asymmetry in vertical cup disc-to-ratio as statistically significant predictors for the development of POAG. In a given participant, the risk of developing POAG increased 12% for every mm Hg an eye was higher than the fellow eye. The risk of developing POAG also increased 50% for every 0.1 difference between eyes in cup-to-disc ratio. Baseline asymmetry in VCD was 0.1± 0.1 SD among participants who developed POAG and 0.07 ± 0.1 SD among participants who did not. It should be noted that cup-to-disc ratio was not adjusted for optic disc area because it was not assessed in either OHTS or EGPS.
The predictive accuracy of all three models was good. The “worse” eye model appears to have the best predictive accuracy based on its low (good) calibration chi-square (1.81) compared to 7.32 and 11.19 for the “means plus asymmetry” model and the “means” model respectively. However, the good predictive accuracy of the “worse” eye mdoel may be partly due to statistical circularity. The “worse” eye was selected using the OHTS/EGPS prediction model which was developed from the same sample. To some extent, the worse eye model becomes a self-fulfilling prophecy and may not perform equally well in an independent sample.
We examined whether the three predictive models would perform equally well in a clinical setting. The “means” model, which has five factors, was the simplest to calculate and yielded the most stable estimate of risk. The “means plus asymmetry” model, which has seven factors, requires calculation of the means of four eye-specific variables and the absolute differences in IOP and VCD. Reliable assessment of asymmetry in IOP and VCD is critical because measurement variability can easily obscure or conflate the true magnitude of asymmetry and result in erroneous calculations of risk. The range of IOP asymmetry was small with 74% of the participants having IOP asymmetry of 2 mm Hg or less and 79% having VCD asymmetry of 0.1 or less. When we reran the “means plus asymmetry” prediction model using only the first of 6–9 IOP measurements per eye to mimic the clinical setting (rather than the mean of 6–9 IOP measurements per eye as done in this study), IOP asymmetry was not statistically significant. We were unable to evaluate the impact of variability in cup-to-disc estimates because only one grading by trained readers using stereophotographs was performed in the OHTS and the EGPS. However, numerous studies have reported differences of 0.2 disc diameters or greater among skilled graders in 13% to 19% of the readings of VCD gradings from optic disc photographs 23–26 These studies suggest that variability in the clinical evaluation of VCD could reduce the reliability of the risk estimate.
We encountered an unexpected limitation in the application of the “worse” eye model in this study. The “worse” eye model requires calculation of risk for each eye using IOP, CCT, PSD and vertical-cup-disc ratio and then the selection of the eye with the higher risk. No one variable could be used to identify the “worse” eye. Only 2 percent (17 of 847) of the participants in this study were found to have a difference between eyes of 20% or greater in the 5-year risk of developing POAG. The application of the “worse” eye prediction model in clinical practice may be limited by the fact many ocular hypertensive individuals may not have an eye that is materially “worse” at baseline. Thus, advantages of the “worse” eye model over the “means” prediction model are not clear.
The OHTS/EGPS prediction model is among the first ophthalmic models to be developed and confirmed in a large, independent sample. As new information about risk factors emerge, the prediction model for the development of POAG will be further refined. We evaluated whether adding asymmetry between eyes at baseline or using the “worse” eye at baseline improved the OHTS/EGPS prediction model, which uses the means of right and left eyes for eye-specific variables. We conclude that the “means plus asymmetry” and “worse” eye prediction models were statistically equivalent to the “means” model, but that the “means” prediction model, which uses the mean of right and left eyes for eye-specific predictors, is the most robust to measurement variability and error.
Acknowledgments
This work was supported by awards from the National Eye Institute, the National Center on Minority Health and Health Disparities, National Institutes of Health (grants EY09341, EY09307), awards to the Department of Ophthalmology and Visual Sciences at Washington University, the NIH Vision Core Grant P30 EY 02687, awards to the Department of Ophthalmology at University of Miami, the NIH Vision Core Grant P30 EY 01480; the European Commission BMH4-CT-96-1598; Merck Research Laboratories, White House Station, New Jersey; Pfizer, Inc., New York, New York and unrestricted grants from Research to Prevent Blindness, Inc., New York, New York.
*Writing Committee: Mae O. Gordon, PhD1; Michael A. Kass, MD1, Valter Torri, MD2; Stefano Miglior, MD3; Julia A. Beiser, MS1; Irene Floriani, PhD2; J. Philip Miller, AB1; Feng Gao, PhD1; Ingrid Adamsons, MD, MPH4; Davide Poli, ScD2. A complete list of personnel is available at https://ohts.wustl.edu/risk
1Washington University, St. Louis, Missouri; 2Istituto di Ricerche Farmacologiche “Mario Negri”, Milan, Italy; 3Policlinico di Monza University of Milano-Bicocca, Milan, Italy; 4Merck Research Laboratories, Blue Bell, Pennsylvania.
Footnotes
Conflict of Interest: No conflicting relationship exists for any author
Meeting Presentation: Presented at American Academy of Ophthalmology Annual Meeting, November 2006, Las Vegas, NV
This article contains additional online-only material. The following appears only online: Organizations and collaborators of the OHTS and EGPS, and the quantitative 5-year prediction model with examples. The material will not appear in the printed version, but will be archived at https://ohts.wustl.edu/risk.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 3.European Glaucoma Prevention Study (EGPS) Group. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 4.Ocular Hypertension Treatment Study Group, European Glaucoma Prevention Study Group. . Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 6.European Glaucoma Prevention Study (EGPS) Group. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366–75. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Gordon MO, Kass MA Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 8.European Glaucoma Prevention Study (EGPS) Group. The European Glaucoma Prevention Study design and baseline description of the participants. Ophthalmology. 2002;109:1612–21. doi: 10.1016/s0161-6420(02)01167-3. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DR, Patella VM. Automated Static Perimetry. 2. St. Louis, MO: Mosby; 1999. p. 115. [Google Scholar]
- 10.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 11.D’Agostino RB, Sr, Grundy S, Sullivan LM, et al. CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 12.Levine RA, Demirel S, Fan J, et al. Ocular Hypertension Treatment Study Group. Asymmetries and visual field summaries as predictors of glaucoma in the Ocular Hypertension Treatment Study. Invest Ophthalmol Vis Sci. 2006;47:3896–903. doi: 10.1167/iovs.05-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quigley HA, Enger C, Katz J, et al. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644–9. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 14.Ong LS, Mitchell P, Healey PR, Cumming RG. Asymmetry in optic disc parameters: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40:849–57. [PubMed] [Google Scholar]
- 15.Jonasson F, Damji KF, Arnarsson A, et al. Prevalence of open-angle glaucoma in Iceland: Reykjavik Eye Study. Eye. 2003;17:747–53. doi: 10.1038/sj.eye.6700374. [DOI] [PubMed] [Google Scholar]
- 16.Harasymowycz P, Davis B, Xu G, et al. The use of RADAAR (ratio of rim area to disc area asymmetry) in detecting glaucoma and its severity. Can J Ophthalmol. 2004;39:240–4. doi: 10.1016/s0008-4182(04)80120-0. [DOI] [PubMed] [Google Scholar]
- 17.Salgarello T, Colotto A, Valente P, et al. Posterior pole retinal thickness in ocular hypertension and glaucoma: early changes detected by hemispheric asymmetries. J Glaucoma. 2005;14:375–83. doi: 10.1097/01.ijg.0000176933.14229.fc. [DOI] [PubMed] [Google Scholar]
- 18.Bagga H, Greenfield DS, Knighton RW. Macular symmetry testing for glaucoma detection. J Glaucoma. 2005;14:358–63. doi: 10.1097/01.ijg.0000176930.21853.04. [DOI] [PubMed] [Google Scholar]
- 19.Vernon SA, Jones SJ. Intraocular pressure asymmetry in a population tested with the Pulsair non-contact tonometer. Eye. 1991;5:674–7. doi: 10.1038/eye.1991.124. [DOI] [PubMed] [Google Scholar]
- 20.Atkin A, Wolkstein M, Bodis-Wollner I, et al. Interocular comparison of contrast sensitivities in glaucoma patients and suspects. Br J Ophthalmol. 1980;64:858–62. doi: 10.1136/bjo.64.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham SL, Klistorner AI, Grigg JR, Billson FA. Objective VEP perimetry in glaucoma: asymmetry analysis to identify early deficits. J Glaucoma. 2000;9:10–9. doi: 10.1097/00061198-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Nicolela MT, Drance SM, Rankin SJ, et al. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol. 1996;121:502–10. doi: 10.1016/s0002-9394(14)75424-8. [DOI] [PubMed] [Google Scholar]
- 23.Tielsch J, Katz J, Quigley HA, et al. Intraobserver and interobserver agreement in measurement of optic disc characteristics. Ophthalmology. 1988;95:350–6. doi: 10.1016/s0161-6420(88)33177-5. [DOI] [PubMed] [Google Scholar]
- 24.Varma R, Spaeth GL, Steinmann WC, Katz LJ. Agreement between clinicians and an image analyzer in estimating cup-to-disc ratios. Arch Ophthalmol. 1989;107:526–9. doi: 10.1001/archopht.1989.01070010540027. [DOI] [PubMed] [Google Scholar]
- 25.Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992;99:215–21. doi: 10.1016/s0161-6420(92)31990-6. [DOI] [PubMed] [Google Scholar]
- 26.Zangwill L, Shakiba S, Caprioli J, Weinreb RN. Agreement between clinicians and confocal scanning laser ophthalmoscope in estimating cup/disc ratios. Am J Ophthalmol. 1995;199:415–21. doi: 10.1016/s0002-9394(14)71226-7. [DOI] [PubMed] [Google Scholar]