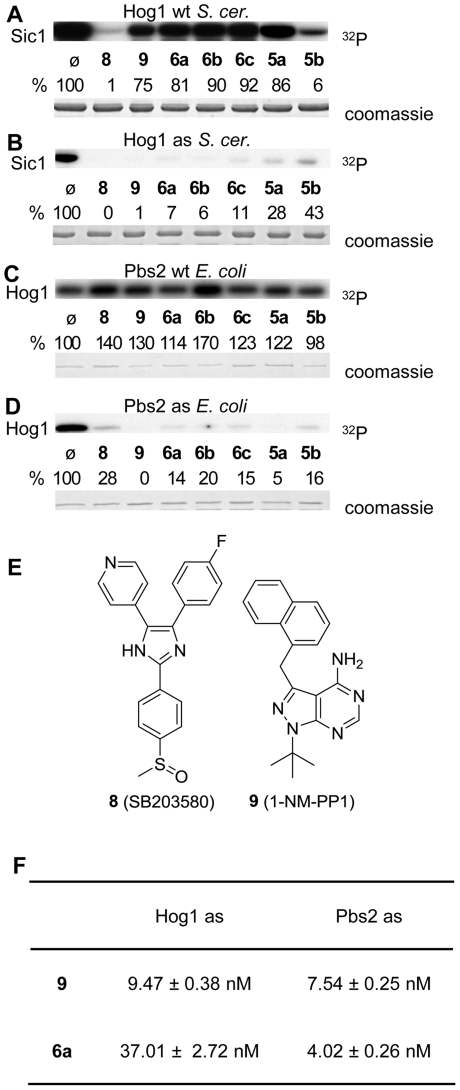
Figure 3. In vitro inhibition of Hog1 and Pbs2.
In vitro inhibition of Hog1as (B) and Pbs2as (D) mutant variants by inhibitors, and effect in the wild type partners (A and C). The inhibitors used [final concentration 5 µM] were 6a–6c, 5a, 5b, as well as SB203580 (8) (a known inhibitor of p38α, p38β, p38β2 and AKT/PKB) and 1NMPP1 (9), a known inhibitor of the as kinases. Recombinant, tagged proteins were purified either from S. cerevisiae (Hog1) or E.coli (Pbs2) and were assayed for the phosphorylation of Sic1 (substrate of Hog1) or Hog1 (as substrate of Pbs2). Phosphorylated proteins were resolved by SDS-PAGE and their phospho-state detected by autoradiography. IC50 values for in vitro inhibition of Hog1as and Pbs2as mutant variants by 6a and 9 (F). Recombinant tagged proteins were purified either from S. cerevisiae (Hog1) or E.coli (Pbs2) and were assayed for phosphorylation of Sic1 (substrate of Hog1) or Hog1 (as substrate of Pbs2). The results are the means ± S.D. of at least three independent experiments.