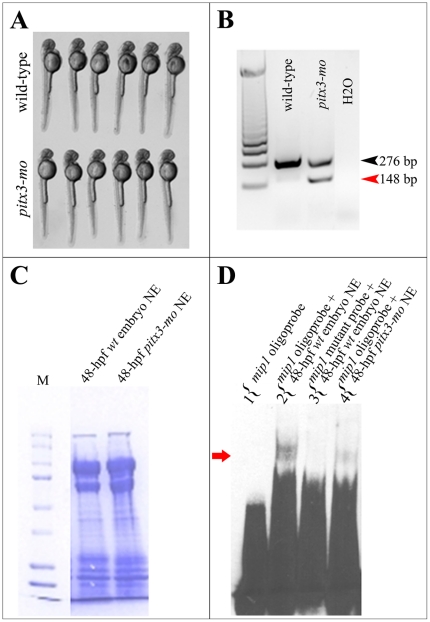
Figure 6. Formation of high molecular weight mip1 promoter- protein complexes is dependent on pitx3 presence.
A. 48-hpf wild-type and pitx3 morphant embryos showing normal body length and morphology that were selected for EMSA experiments. B. Results of RT-PCR performed with RNA extracted from the pooled tail tissues from wild-type and pitx3 morphant embryos shown in C. A sharp reduction in normal pitx3 transcript (black arrowhead) and the presence of abnormally spliced product (red arrowhead) are evident in pitx3 morphant embryos. C. Coomassie Blue R-250 stained polyacrilamide gel demonstrating equal protein concentration in nuclear extracts obtained from wild-type (lane 1) and pitx3 morphant (lane 2) embryos that were used in EMSA experiments shown in A. D. Electrophoretic mobility shift assays (EMSA) show formation of a DNA-protein complex when an oligonucleotide corresponding to the −44/−76 region of zebrafish mip1 promoter and nuclear extracts from 48-hpf wild-type zebrafish embryos are used. Please note a presence of a specific slow migrating complex, which is formed by wild-type mip1 probe and proteins extracted from nuclei of 48-hpf wild-type zebrafish embryos (lane 2), absence of this complex in lane 3 when the same nuclear extracts were combined with a mutant mip1 probe where the pitx3-binding bicoid site GGATTA was replaced by AAATTA, and sharp reduction of this complex in lane 4 containing a combination of a wild-type mip1 probe and nuclear extracts obtained from pitx3 morphants (red arrow).