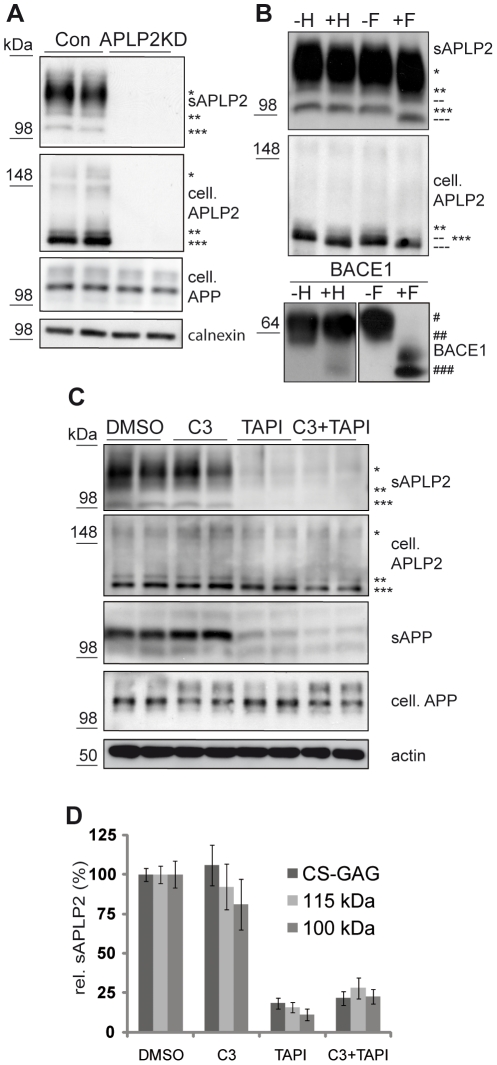
Figure 1. Analysis of APLP2 shedding using protease inhibitors.
(A) Human neuroblastoma SH-SY5Y cells were transiently transfected with an siRNA pool against APLP2 or with a control pool. Endogenous APLP2 was detected with the N-terminally binding 2D11 antibody. Bands were absent upon knock-down of APLP2 (APLP2KD), demonstrating the specificity of the antibody. Soluble APLP2 (sAPLP2) was detected in the conditioned medium, cellular full-length APLP2 (cell. APLP2), cellular full-length APP (cell. APP) and calnexin as a loading control in the cell lysate. Three forms of APLP2 were detected: CS-GAG modified (*) and two non-CS-GAG modified species (** and ***) with molecular weights of around 115 and 100 kDa respectively. (B) Deglycosylation of APLP2 in conditioned medium and cell lysate of SH-SY5Y cells using endoglycosidase H (H) and N-glycosidase F (F). Deglycosylated forms of APLP2 are indicated (-- and ---). As a control, deglycosylation was performed for BACE1 in the cell lysate of BACE1 overexpressing HEK293 cells. Mature (#), immature (##) and deglycosylated (###) BACE1 is detectable. (C) Representative blots of treatment of SH-SY5Y cells with C3 (1 µM), TAPI-1 (50 µM) or C3+TAPI-1. Upon C3 treatment no significant reduction of sAPLP2 levels was observed. Upon TAPI-1 and C3+TAPI-1 treatment sAPLP2 levels were strongly reduced while no changes in APLP2 levels in the cell lysate were observed. As a control, soluble APP (sAPP) levels were clearly reduced upon TAPI-1 and C3+TAPI-1 treatment. (D) Quantification of experiments in C (mean +/− SEM). C3 treatment did not lead to a significant reduction in sAPLP2 levels while TAPI-1 as well as C3+TAPI-1 treatment led to a significant reduction in sAPLP2 levels (p<0.001 for all three species, n = 6).