Abstract
Background
Relapse risk factors, such as psychological stress and alcohol cues, are often encountered together. Understanding how they interact has the potential to improve alcoholism treatments. The present study was conducted to examine whether an acute psychosocial stressor enhanced alcohol cue reactivity in non-treatment-seeking alcoholics.
Methods
Seventy-nine alcohol dependent individuals (39 women) randomly received either the Trier Social Stress Test or a no-stress control condition. Stress reactivity was measured with serum ACTH and cortisol, mean arterial blood pressure, and subjective distress. Immediately following the stress manipulation, participants held and sniffed a neutral cue then their preferred alcoholic beverage. Cue reactivity was measured by two subjective measures of craving following each cue. Additionally, general craving was assessed with the Alcohol Urge Questionnaire (AUQ) at the beginning and end of the laboratory procedure.
Results
The stress manipulation showed internal validity on all measures of stress reactivity. There was not a main effect of stress nor a stress x cue interaction on either cue reactivity measure. As expected, there was a main effect of cue (alcohol > neutral cue) on both measures of cue reactivity. General craving increased during the challenge, but not differently by stress group. Magnitude of stress reactivity was not associated with magnitude of cue reactivity, and all results were independent of gender.
Conclusion
In this well-controlled clinical laboratory study of non-treatment-seeking alcoholics, an acute psychological stressor did not make an alcohol cue a more potent urge-inducing stimulus, and stress had no effect on general alcohol craving.
Keywords: alcohol, stress, craving, cue reactivity, gender
Stress, reminders of alcohol (cues), and a priming drink are each considered potent risk factors for relapse in alcoholics. They have been shown to induce alcohol seeking and drinking in preclinical models (see Epstein and Preston, 2003 for a review), and in clinical samples, they are frequently cited as reasons for relapse (Brown et al., 1995; Litman et al., 1983; Niaura et al., 1988; Zywiak et al., 2003). Each of these factors may have relapse-inducing effects when singularly presented, but individuals attempting abstinence usually do not encounter them separately. A stress-inducing situation (e.g. a stressful day at work) might followed by an alcohol cue (e.g., sight of a bar). Certainly, a priming drink is always encountered in the presence of an alcohol cue (the sight and scent of alcohol). The question of interest in the present study is whether exposure to a stressor makes an alcohol cue more powerful to induce drinking.
In clinical laboratory studies, craving or urge to drink is often used as index of the relapse-inducing power of a stimulus. For example, several studies have examined whether stress increases craving in alcoholics. The results from these studies are surprisingly mixed, likely due to methodologic differences in how stress was induced and how craving was measured. Some have found that a stressor increases alcohol craving in alcoholics (Coffey et al., 2006; Cooney et al., 1997; Fox et al., 2007; George et al., 2008; Sinha et al., 2009), while others have not (Brady et al., 2006; Jansma et al., 2000; Pratt and Davidson, 2009; Rubonis et al., 1994).
If the goal is specifically to determine whether a stressful experience makes an alcohol cue a more powerful urge-inducing stimulus, craving induced by an alcohol cue must be compared to craving induced by a neutral cue (e.g., water). That is, without a control cue, it is not possible to determine whether stress enhances alcohol cue reactivity or simply induces urge to drink (regardless of the presence of cues). Cue reactivity paradigms, such as the one developed by Monti and colleagues (1987), afford the opportunity to rigorously examine this issue (Drummond, 2000).
Relatively few studies have been conducted in alcoholics using a controlled cue exposure paradigm to examine how a stressor affects alcohol cue reactivity. Litt and colleagues (1990) studied a small sample of male inpatient alcoholics with a guided imagery procedure to induce negative mood. They found no evidence that negative mood enhanced alcohol cue reactivity, but this may have been due to lack of power (sample size=8). In addition, negative mood (the stressor) was amenable to confirmation only with subjective report. Also, the investigators did not detect that alcohol cues induced greater urges than water cues, even under neutral mood conditions. That is, the internal validity of cue exposure paradigm was not supported.
Cooney and colleagues (1997) used a similar guided imagery procedure to induce both negative mood and neutral mood in 50 male treatment-seeking alcoholics. Participants were then exposed to both control cues and alcohol cues under each mood condition. The cue exposure procedure differed from the one used by Litt and colleagues (1990) in that participants were re-exposed to the guided imagery procedure between the first and second cue. Desire for alcohol and confidence in one’s ability to resist were the primary measures for cue reactivity. Again, results showed that negative mood did not potentiate alcohol cue reactivity (there was no interaction between mood and cue type), though each factor alone showed a main effect on desire for alcohol (negative mood > neutral mood and alcohol cue > neutral cue). As only men were included in the study, it is unknown whether alcohol dependent women might show stress-potentiated alcohol cue reactivity. Clinical studies (Lau-Barraco et al., 2009; Rubonis et al., 1994) and retrospective reports of reasons for relapse (Connors et al., 1998; Rubin et al., 1996) suggest that women might be more likely than men to show this effect.
Most recently, Mason and colleagues (2008) used images from the International Affective Picture System (IAPS) to induce positive, neutral, and negative mood in 47 non-treatment-seeking abstinent alcoholics (12 women, 26%), followed by exposure to control and alcohol cues. Similar to previous reports, they found main effects of cue type (alcohol > water) on self-reported craving, but neither positive nor negative mood induced by IAPS images potentiated alcohol cue reactivity. Mood induction was confirmed only with subjective measures (where both negative and positive pictures > neutral pictures on induction of “strong emotions”), and the authors note that the pictures used in the IAPS to induce negative affect are not especially relevant to drinking (e.g., a snake about to strike, pictures of physical injuries). Also, while well-powered to test the primary hypothesis, the study did not include a sufficient number of women to afford the opportunity to examine the impact of gender.
Whether and how stress and cues interact is relevant to the real-world threats to recovery that alcoholics face, and better understanding these interactions could improve alcoholism treatments. If stress enhances the power of alcohol cues to induce urge to drink, treatments such as cue exposure therapy might be more effective if patients are repeatedly exposed to alcohol cues following the induction of a stress response. While the extant literature does not support that stress enhances the strength of alcohol cues compared to control cues in clinical samples, limitations of these studies have left open the possibility that if actual stress reactivity is induced and/or if women are included, different results might be obtained. The current study included equal numbers of men and women and utilized a stressor amenable to both objective and subjective confirmation of stress induction. The primary hypothesis tested was that in alcohol dependent individuals, exposure to a standardized psychosocial stressor would enhance alcohol cue reactivity following a standardized cue exposure paradigm. Secondary hypotheses were that the stressor would increase general craving compared to the no stress condition, and that the effects of stress on alcohol cue reactivity and craving would be stronger in women than men.
METHODS
Study Design
A randomized 2 × 2 × 2 mixed model design was employed. The between groups factors were stress exposure (acute psychosocial stressor vs. no stressor) and gender. The repeated measure was cue type (water vs. preferred alcoholic beverage). The decision to use a between groups rather than a within subjects design for the stress manipulation was based on unpublished pilot data that showed that a within subjects design resulted in contaminating expectancy effects regarding the stress manipulation. All participants were compensated for their participation. The study was approved by the university’s institutional review board and was conducted in compliance with the Declaration of Helsinki and the recommendations of the National Advisory Council on Alcohol Abuse and Alcoholism (2005).
Participants
Non-treatment-seeking alcoholics, ages 21 to 65 yrs, were recruited from the community via advertisements. The present study is one of a set of studies in which stress reactivity data will be collapsed across participants to examine gender differences. In one of those studies, alcohol is administered, and so for ethical reasons, we elected to enroll only non-treatment-seekers in both studies.
Following a telephone screening, individuals who were potentially eligible for the study were scheduled for an in person visit, at which time informed consent was obtained. The informed consent form was explicit regarding the procedures of the study and the risks associated with participation, but it was deceptive in the reason for the study. That is, participants were told that the study was conducted to examine the relationship between personality and drinking. They were told that they might be randomly selected (a 50% chance) to complete a “behavioral measure of how outgoing you are and a mental math test to help us collect information about your working memory.” We deemed the deception necessary to avoid demand effects that would be introduced if participants were instructed that they might receive a “stress test.” There was no mischaracterization about the tasks they would be performing in the challenge.
Assessment
Individuals completed a battery of tests and clinical interviews, including the Timeline Followback to assess quantity and frequency of drinking for the prior 30 days (Sobell and Sobell, 1992), the Alcohol Dependence Scale (Skinner and Allen, 1982), State/Trait Anxiety Inventory (Spielberger, 1983), Anxiety Sensitivity Index (Reiss et al., 1986), Beck Depression Inventory II (Beck et al., 1961, 1996), Drinking Motives Questionnaire (Cooper et al., 1992), Barrett Impulsiveness Scale II (Patton et al., 1995), and the Neo Five Factor Personality Inventory (Costa et al., 1992). A clinical interview was conducted using the MINI (Sheehan et al., 2002) to confirm alcohol dependence diagnosis and to rule out exclusionary Axis I disorders using DSM-IV criteria.
To be eligible for the study, participants had to meet diagnostic criteria for current alcohol dependence and had to drink at least 14 drinks (women) or 20 drinks (men) per week. Participants were excluded if they had other current major Axis I disorders, including current dependence on other drugs (excepting nicotine), were taking any psychotropic medications or medications known to affect the HPA axis, had a body mass index > 35, expressed interest in receiving treatment for alcoholism, or had received prior medical alcohol detoxification.
Participants who remained eligible following assessment were scheduled for their second (challenge) visit, which was conducted at the General Clinical Research Center of MUSC. Cycling women were scheduled for the challenge visit during days 1–10 following menstruation (follicular phase), when estradiol and progesterone are relatively low. Women using oral contraceptives were not excluded from participation and were scheduled for testing at the same point as free-cycling women.
Prior to completing the assessment visit, participants were instructed to abstain from alcohol for 48 hours prior to the challenge, to fast after noon, and to avoid caffeinated beverages on the day of the challenge. They were informed that they would receive both a breath alcohol test and a urine drug screen on the day of the challenge and if either test was positive, they would be excluded and would forfeit compensation for their participation.
Laboratory Procedure
Table 1 shows the challenge visit timeline. The challenge visit was conducted on average 21 days after the initial visit. All participants were tested individually. Participants arrived at the GCRC at 4:00 pm, and eligibility criteria were evaluated, including absence of marked alcohol withdrawal (5 or less on the Clinical Institute Withdrawal Assessment of Alcohol Scale, Sullivan et al., 1989). An indwelling catheter was placed in the non-dominant arm at 4:20 pm. Two baseline stress assessments were collected prior to the stress manipulation. From 5:00 to 5:15 pm, participants received either the stressor (described below) or the no-stress control condition (reading a travel magazine alone for 15 min). Immediately following (from 5:20 to 5:30), all participants received the cue exposure paradigm. At 6:30 pm, participants were compensated and provided with a feedback report of results from the psychological test battery conducted at their first visit, including information about quantity and frequency of drinking, and information about available alcohol treatment services.
Table 1.
Timeline of activities in the challenge procedure
| Time | Procedure | Data |
|---|---|---|
| 4:00 to 4:15 | Participant arrives, assessed for compliance/safety |
Self-reported last drinking day > 2 days prior; breath alcohol test, uring pregnancy, urine drug screen, CIWA-AR ≤ 5 |
| 4:15 | Pre-challenge craving assessed | Alcohol Urge Questionnaire |
| 4:20 | Indwelling catheter placed | |
| 4:40 | Baseline stress assessment | Cortisol, ACTH, SUD, MAP |
| 4:55 | Baseline stress assessment | Cortisol, ACTH, SUD, MAP |
| 5:00 to 5:15 | TSST or no stress control | |
| 5:15 to 6:15 | Post-stress assessments (n=7) | Cortisol, ACTH, SUD, MAP |
| 5:20 | Cue exposure-Water | VAS (craving), Market value (craving) |
| 5:25 | Cue exposure-Alcohol | VAS (craving), Market value (craving) |
| 6:15 | Post-challenge craving assessed | Alcohol Urge Questionnaire |
| 6:30 | Participant discharged |
Stress Induction
The Trier Social Stress Test (TSST) (Kirschbaum et al., 1993) is an acute psychosocial stressor consistently shown to induce a marked stress response in men and women (Kudielka et al., 2007). It contains two critical elements for inducing psychological distress, a social evaluative component (public speaking) and a performance component (mental math) (Dickerson and Kemeny, 2004).
At 5:00 pm, participants randomized to receive the TSST were instructed that they would soon complete the behavioral personality test to an audience with expertise in assessing body language and behavior. Paper and a pen were provided, and each participant was instructed to prepare to give a speech to three audience members on why s/he should be hired for a “dream job.” A countdown clock set to 5 min was set in front of the participant, and s/he was left alone to prepare. Once 5 min elapsed, the three audience members (confederates) in white lab coats entered the room. One was a spokesperson, who instructed the participant to stand and begin. Confederates remained stoic during the speech; when the participant paused for more than 10 sec, the spokesperson said, “Your time is not up; please continue.” Following 5 min of public speaking, the participant was told that the interview was complete and to begin the mental math test by serially subtracting 13 from 1022. Following every incorrect response, the participant was instructed to “begin again at 1022.” After 5 min of the mental math test, the spokesperson instructed the participant to return to his/her seat, and all confederates left the room.
Cue Exposure
The alcohol cue exposure procedure employed was developed by Monti and colleagues (1987). Each participant held and sniffed a control beverage (spring water) for 3 min, followed by measurement of cue reactivity. After a 3-min rest period, the participant’s preferred alcoholic beverage was presented and poured into an appropriate glass (wine glass for wine; pint glass for beer). The participant held and sniffed the contents of the glass for 3 minutes, and then again completed the cue reactivity assessments. Order of cues was not counterbalanced since carryover effects may occur when alcohol cues are presented first.
Laboratory Assessments
Stress Reactivity
Stress reactivity was assessed with four measures: adrenocorticotropic hormone (ACTH) (pg/ml), serum cortisol (µg/dl), mean arterial pressure (mm/Hg), and subjective distress (self-reported level of distress on a 0–10 scale). Neuroendocrine measures were collected twice at baseline to obtain a stable index, and at 0 (5:15 pm), 5, 10, 20, 30 (ACTH and cortisol) and 45 and 60 min (cortisol only) post-stressor. Blood draws for ACTH were truncated at 30 min, as ACTH typically returns to baseline levels by this time (Kirschbaum et al., 1993).
Blood samples for ACTH and cortisol were collected in iced EDTA tubes, plasma was separated from cells by centrifugation, and the serum sample was frozen at −70°C until thawed for assay. ACTH was measured by the IMMULITE 2000 ACTH test (Siemens Healthcare Diagnostics, Flanders, NJ). Preparation, set-up, dilutions, adjustment, assay and quality control procedures were performed according to the operator’s manual. At 28 pg/ml, the intra-assay coefficient of variation (cv) was 1.8%. The functional sensitivity (lowest reportable concentration) was 5.9 pg/ml. Cortisol was assayed using the ADVIA Centaur XP immunoassay system (Siemens Healthcare Diagnostics, Flanders, NJ). Functional sensitivity was 0.2 µg/dl and intra-assay cv was 2.15% at 44 ug/dl.
Cue Reactivity
Physiologic measures of cue reactivity (e.g., salivation, heart rate, blood pressure) are confounded by the TSST (pilot data, unpublished), and so were not used in the present study to index cue reactivity. Cue reactivity was measured by two subjective reports following each cue. Participants reported their “craving for alcohol right now” on a visual analog scale (0–100). In addition, a novel measure of alcohol desire developed by our group was used. During the initial assessment visit, participants were asked how much they typically pay for one serving of their preferred alcoholic beverage (which also served as the beverage for the alcohol cue in the challenge). Following exposure to each cue, participants were asked, “You usually pay [exact dollar amount] for one serving of [preferred alcohol]; if you could have one NOW, how much would you pay for it?” Responses were divided by the cost reported in the initial assessment interview to compute a “market value” measure. For example, a score of 2.0 reflects that the participant reportedly would pay double what s/he typically pays.
General Alcohol Craving
The Alcohol Urge Questionnaire (AUQ, Bohn et al., 1995) was administered at the beginning (4:15pm) and end (6:15pm) of the challenge procedure. The AUQ is a validated instrument (range=8–56) for assessing state craving (Drummond and Phillips, 2002). It has a single factor structure and has been successfully used for real-time measurement of alcohol craving (MacKillop, 2006).
Data Analysis
Stress reactivity data were summarized to confirm the internal validity of the stress manipulation. Complete characterization of participants’ stress response curves (and the effect of gender) is the focus of a separate paper. In the present report, difference scores were computed for subjective distress and mean arterial pressure by subtracting mean baseline scores from the first post-stress measurement. For ACTH and cortisol, area under the curve was computed and used in analyses. Analysis of variance and covariance were used to compare stress vs. no stress groups on these four measures of stress response.
For alcohol cue reactivity, VAS craving rating (0–100) and market value following each cue type were analyzed with repeated measures analysis of variance; factors were stress, cue type, and gender. For general craving, pre and post-challenge AUQ scores were analyzed with repeated measures ANOVA. Factors were stress, time, and gender.
RESULTS
Participants
In total, 333 telephone screenings were conducted; 109 individuals were invited for an in person interview and signed informed consent; 84 met all inclusion criteria; and 80 subjects participated in the challenge. Of the four individuals who did not participate in the challenge, two declined, and two had a positive breath alcohol reading on the day of the challenge.
One woman in the stress group became extremely agitated during the stressor. She removed her blood pressure cuff and provided outlying responses (> 2 SD above stress-group mean) on all self-report measures. Her data were considered unreliable and were excluded from outcome analyses. The resulting sample size was 39 women (20 no stress/19 stress) and 40 men (19 no stress/21 stress).
Table 1 shows demographic, drinking, and psychological assessment data by stress group and gender. Despite random assignment to groups, the no stress group > stress group on age, percent married, and severity of trait anxiety (STAI), ps < 0.05. These variables were included as covariates in all analyses of the craving and cue reactivity measures, but were dropped if no significant main or interaction effects were revealed. Participants drank on average 50 drinks per week, and between 9 and 10 drinks per drinking day. Alcohol dependence severity (M=13) was in the moderate range. Quantity and frequency of alcohol use was not assessed in the interim between the initial visit and challenge visit. We asked participants to report date of last drink when s/he arrived for the challenge visit to confirm compliance with 48 hour requested abstinence. Mean number of days since last drink was 3.0 (SD=1.5), with no stress group or stress group x gender effects.
Stress induction
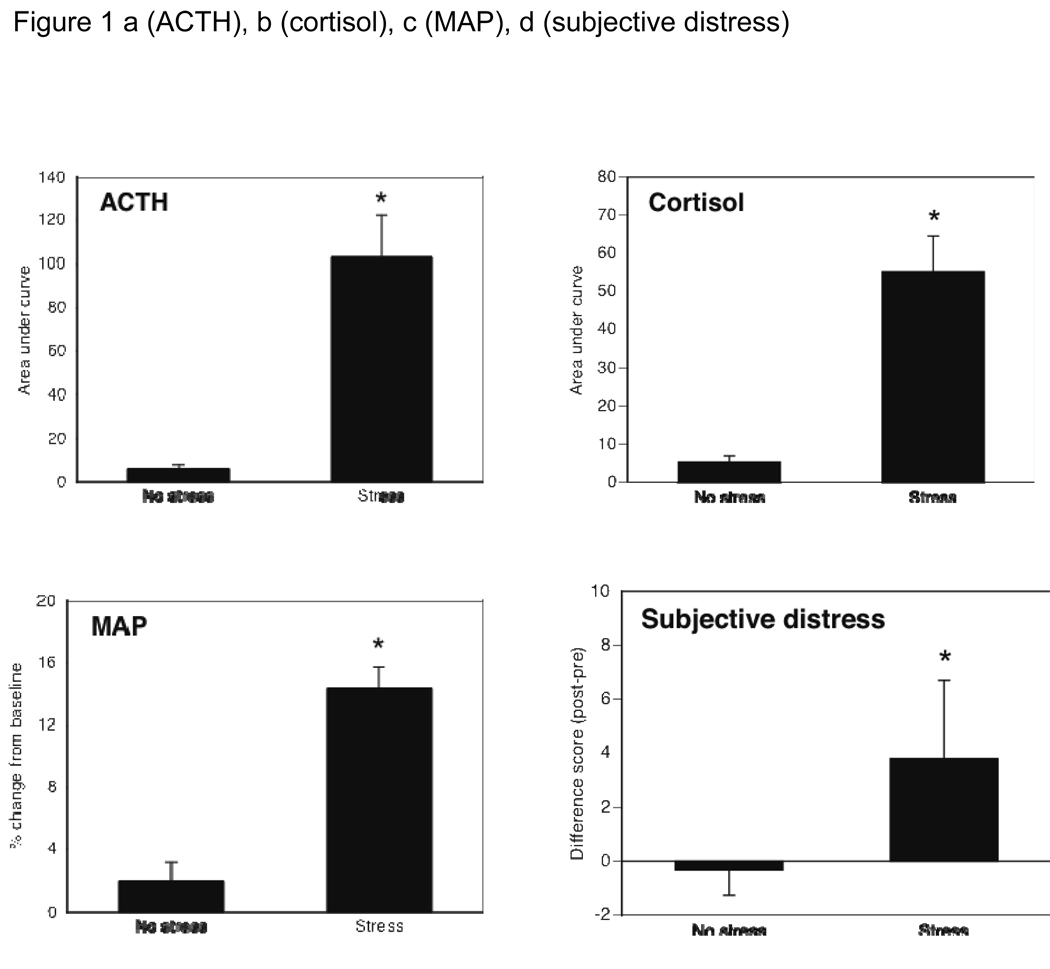
Stress and no-stress groups were compared on mean baseline values for the four primary stress indices. The two groups were similar on baseline subjective distress (M=1.8, SD=1.8), ACTH (M=15.9 pg/ml, SD=18.5), and cortisol (M=10.9 µg/dl, SD=4.9), all p values > .10. By chance, the TSST group had slightly lower baseline mean arterial pressure (MAP) (M=81.5 mm/Hg, SD=10.6) than the control group (M=88.6 mm/Hg, SD=15.1), p=0.02, and so MAP was covaried. ANCOVAs confirmed the stress manipulation had internal validity, producing a robust response in all measures of stress reactivity (all F values > 22.0, all ps<0.001). Effect sizes (Cohen’s d) were large, ranging from 1.1 for ACTH to 1.9 for subjective distress. Figure 1a–d shows group means on each stress response index. Gender differences were observed on some measures of stress reactivity, but those are outside the scope of the present paper.
Figure 1.
ACTH (Fig 1a), cortisol (Fig 1b), mean arterial blood pressure (Fig 1c), and subjective distress (Fig 1d) each showed a significant effect of the stress manipulation, confirming the internal validity of the stressor with both objective and subjective measures.
Alcohol cue reactivity
Main effects
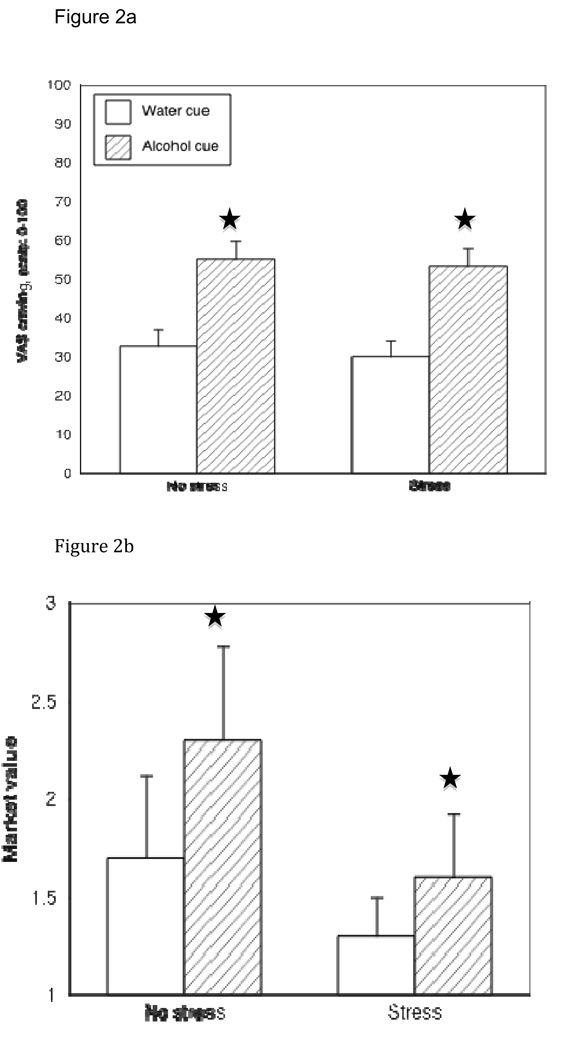
There was a significant main effect of cue type on both VAS craving ratings and market value, where both measures were higher following the alcohol cue than the control cue [Fs(1,75)>16.0, p values <.001], thereby confirming the internal validity of the cue exposure paradigm. It also supports that the market value measure may be a useful addition to the standard battery of assessments to determine changes in craving following cue exposure. There was not a main effect of stress or gender on either measure of cue reactivity (p values >.30).
Interaction Effects
There was no three-way interaction between cue type, stress group and gender for either cue reactivity measure (both p values >.20). The interaction between stress group and cue type was also not significant for either VAS (F(1,75)=.10, p=.76) or market value (F(1,75)=1.62, p=.21). Figure 2a and 2b show mean response to water and alcohol cues by stress group (bars show SEM) for these outcomes. Age, marital status, and STAI scores were covaried in analysis of both variables and in no case was the conclusion altered. Adjusted means differed only slightly from the unadjusted values shown in the figures.
Figure 2.
VAS (Fig 2a) and Market value (Fig 2b) scores following exposure to the control cue and the alcohol cue in each stress group. For both measures, alcohol > water cue, p<0.001. There was neither a main effect of stress nor an interaction between cue type and stress on either measure, reflecting that stress exposure did not affect alcohol cue reactivity.
General craving
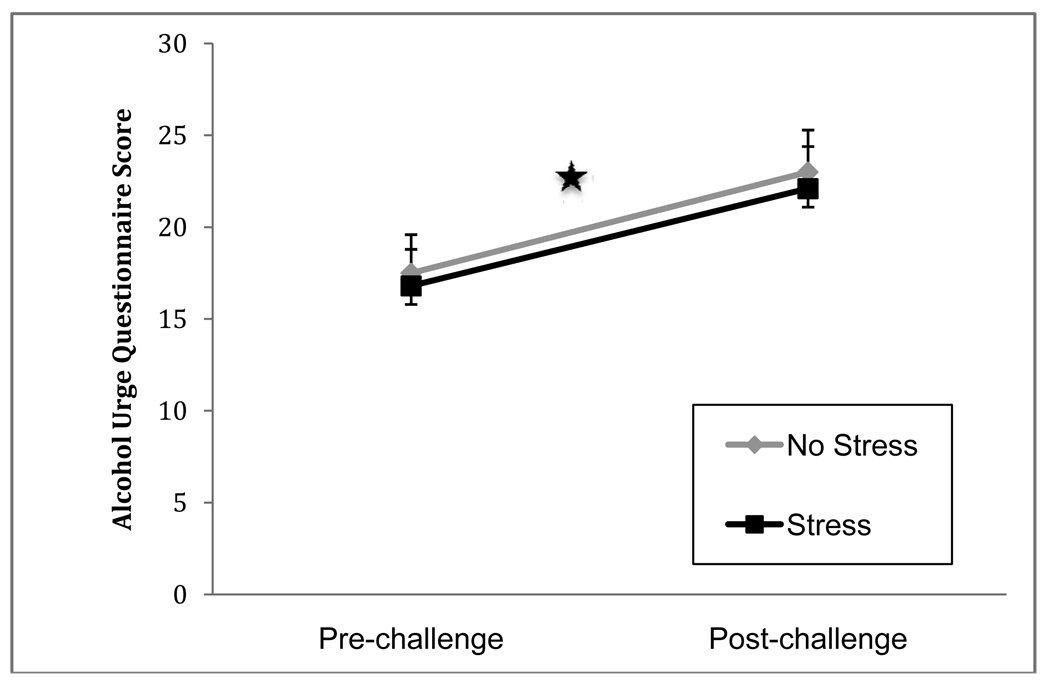
The AUQ was administered at the start and end of the challenge to index overall state craving. There was a significant effect of time (post > pre), F(1,75)=20.24, p=.001 (d=2.48). There were no main effects of gender or stress group on AUQ scores (both ps>.60). There was not a significant three way interaction between time x stress group x gender, F(1,75)=0.87, p=0.35, nor was there a time x stress group effect, F(1,75)=.007, p=.93, indicating that the rise in AUQ scores during the challenge was not affected by exposure to the stressor. Neither age, marital status, nor STAI as covariates changed the estimate of the effects of stress. Unadjusted means are shown in Figure 3.
Figure 3.
Mean Alcohol Urge Questionnaire scores by stress group before and after the clinical laboratory challenge. All participants received the alcohol cue exposure paradigm; half received the TSST beforehand. AUQ scores increased pre vs. post challenge p<0.0001, but not differently by stress group.
Exploratory analyses
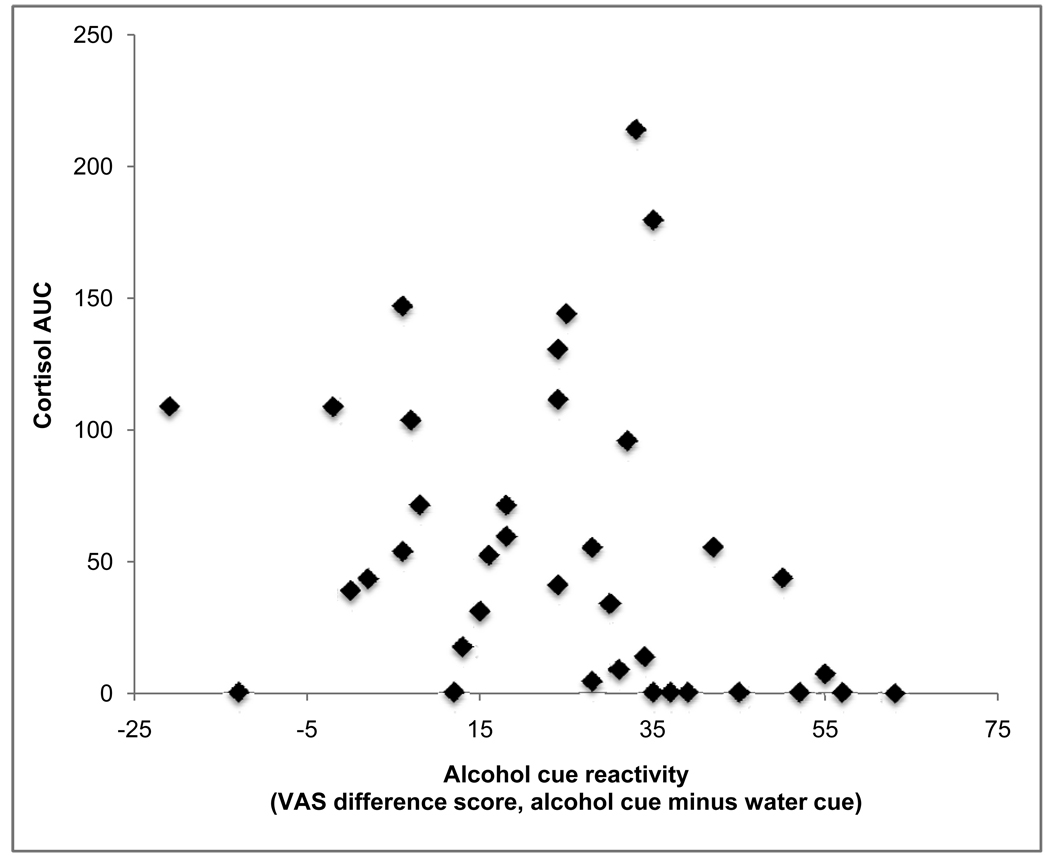
Limiting analyses only to participants who received the TSST, there was marked variability in stress response. Correlation analyses were conducted to examine whether stress reactivity was related to enhanced cue reactivity or increased craving (via AUQ) following the cue exposure procedure. Specifically, difference scores (response to alcohol minus response to water) were used for VAS craving and for market value to index changes in cue reactivity, and for AUQ, the difference score from post-test to pre-test was used to index changes in craving. Area under the curve for cortisol and ACTH were used to index neuroendocrine reactivity, and difference in subjective response from baseline to first post-stress measure was used to index subjective stress reactivity. Percent change from baseline to first post-stress measurement was used to index change in mean arterial pressure. All distributions were normal. In no case did any of the stress reactivity measures correlate significantly with any of the cue reactivity or craving measures (r values ranged from −0.24 to 0.26, all p values > 0.10). Figure 4 is a representative scatterplot and shows VAS craving difference scores (alcohol cue minus water cue) by cortisol area under the curve.
Figure 4.
In participants who received the TSST, there was no association between magnitude of stress reactivity (figure shows cortisol area under the curve) and alcohol cue reactivity (alcohol minus water difference score on VAS craving).
We also explored whether pre-existing traits conferred susceptibility to stress-enhanced alcohol cue reactivity or general alcohol craving—that is, in whom, if anyone, was the hypothesis supported? These analyses were based on the extant literature that anxiety sensitivity (Stewart et al., 1997), dependence severity (Rohsenow et al., 1992), coping motives for drinking (Otto et al., 2004), impulsivity (Drummond, 2000) and/or neuroticism (McCusker and Brown, 1991) might be traits of interest. Each of these was measured with a psychometrically-validated instrument (see Methods). Note that all prior analyses of cue reactivity and craving outcomes already included age, marital status, and scores on the STAI as covariates without effect. Whether entered into an ANCOVA as continuous variables or as dichotomous variables (via median split), none of the traits above predicted baseline-controlled outcome variables in the TSST group (all p values > 0.15). Further, inclusion of these variables as covariates in ANCOVAs conducted on the entire sample (with stress group and gender as between groups factors) did not alter any of the aforementioned conclusions. In summary, these variables, while related to stress reactivity and/or cue reactivity in the literature, did not confer risk for stress-potentiated cue reactivity or craving in this sample of non-treatment-seeking alcoholics.
DISCUSSION
This clinical laboratory study examined whether exposure to an acute psychosocial stressor enhanced craving induced by an alcohol cue (compared to a control cue) in non-treatment-seeking alcoholics. While there was clear evidence that the manipulations worked as expected (the stressor induced a stress response and the alcohol cue induced greater craving than the control cue), there was not an interaction between stress and cue type on cue reactivity, and this result was consistent for both genders. Results from the present study and others (Cooney et al., 1997; Litt et al., 1990; Mason et al., 2008) argue that if stress does induce urge to drink, it does not appear to do so by making alcohol cues more potent.
In fact, the question of whether stress induces craving in alcoholics warrants more research attention. Results from clinical laboratory studies are equivocal, with a near equal number of positive (Coffey et al., 2006; Cooney et al., 1997; Fox et al., 2007; George et al., 2008; Sinha et al., 2009) and negative studies (Brady et al., 2006; Jansma et al., 2000; Pratt and Davidson, 2009; Rubonis et al., 1994). The present study adds to the latter group, as there was no main effect of stress on any of the three craving measures (both cue reactivity measures and general craving as measured by the AUQ). While AUQ scores increased over the course of the challenge, these did not differ by stress group. Furthermore, the magnitude of stress reactivity in those who received the stressor was not associated with degree of alcohol cue reactivity nor change in AUQ scores following the challenge.
There is substantial evidence that alcoholics have altered HPA axis response to stressors (Adinoff et al., 2005; Costa et al., 1996; Lovallo et al., 2000), and it has been proposed that normalizing the stress response or reducing stress reactivity, either by medication or behavioral treatment, might reduce risk of relapse (Coffey et al., 2006; Kiefer et al., 2006; O’Malley et al., 2002; Stasiewicz et al., 1997). If stress responsivity is related to relapse, it may be that a perturbed stress response confers this risk by impairing one’s ability to inhibit a prepotent response, rather than by increasing craving. This possibility is suggested by a recent study in non-alcoholic participants, where greater cortisol response to the TSST was associated with more impulsive choices on a gambling task, but only in men (van den Bos et al., 2009).
Undoubtedly, the stressor one uses in clinical laboratory studies impacts the results. We utilized the Trier Social Stress Test because it is most amenable to objective confirmation of the stress response. Guided imagery has also been used in this area of research as an acute stressor, an approach that has the benefit of personalizing the stressor to each participant. The majority of studies showing that stress induces urge to drink in alcoholics have used guided imagery stressors (see Sinha, 2008 for a review). Guided imagery differs considerably from the TSST, in that the former attempts to induce a stress response by having the participant re-imagine a stressful personal experience, whereas the TSST requires the participant to engage in activities that are commonly considered anxiety provoking (public speaking and mental arithmetic). These procedures, as well as other stress induction methods, likely induce different kinds of affective distress, so we can not conclude that our results generalize to all stressful experiences. Notably though, two of the three studies that have failed to find stress-induced potentiation of craving to alcohol cues (Cooney et al., 1997; Litt et al., 1990) used guided imagery to induce stress, thus suggesting that the negative results of this study are not solely due to the specific stressor utilized.
In addition to different stressors inducing different subjective responses, there are likely substantial individual differences in subjective response to the TSST. Upon completing the task, some individuals may experience relief, while others experience sustained anxiety, and others experience anger. Subjective response to the stressor was measured broadly in the present study (rate your level of distress), and so these individual differences in emotions to the stressor were not assessed. It is possible that if we had asked about specific emotions, we may have found a subset of individuals (e.g., those who experience anger to the stressor) who showed stress-potentiated alcohol cue reactivity and/or craving.
Cue reactivity and craving were assessed exclusively with self-report measures, which a recent review concludes are the best and most valid approach for measuring craving (Rosenberg, 2009). Others have shown that implicit or autonomic measures of urge may be more sensitive and accurate reflections of changes in craving (Rohsenow et al., 1992; Sayette et al., 2000), leaving open the possibility that stress could potentiate craving or cue reactivity outside of conscious awareness, as suggested by Tiffany’s (1990) model of automaticity. It is notable that all measures of cue reactivity in the present study showed the hypothesized cue effect (alcohol > water), and scores on the AUQ were also increased following the challenge procedure, in which all participants were exposed to an alcohol cue. Mean scores on the scaled instruments of craving (VAS and AUQ) were each around 55% of maximum. Because others have found that self-reported craving induced by drug cues can clearly exceed this (Carter and Tiffany, 1999; MacKillop, 2006), ceiling effects in these measures were unlikely. Taken together, these results suggest that our reliance on self-reported measures of craving, while limiting our ability to capture the multidimensional nature of craving, did not hinder our ability to examine whether an acute stressor potentiated the effects of an alcohol cue on conscious craving.
We found no evidence that an acute stressor differentially altered craving or cue reactivity by gender in our participants. Though not shown, means on all cue reactivity and craving measures were tightly clustered for women and men within each stress group, reflecting that the absence of an effect was not likely due to lack of power to detect meaningful differences. This result was contrary to the hypothesized effect, though studies conducted in social drinkers have also failed to find that women are more susceptible to stress-induced alcohol craving. Nesic and Duka (2006) found that stress prevented galvanic skin response to alcohol cues in women but not men, and stressed women showed lower subsequent alcohol consumption than stressed men. No control cues were used in the study, which prevents conclusions to be drawn regarding alcohol cue reactivity. Chaplin and colleagues (2008) found that in social drinkers, subjective response to stress and alcohol cue scripts were both linked with craving in men but not women. We hypothesized that stress would have a greater effect on cue reactivity and craving in women based on the extant literature from treatment-seeking samples, where women endorse drinking to stress-related situations more commonly than men do (Lau-Barraco et al., 2009) and showed greater cue-elicited craving following negative mood induction (Rubonis et al., 1994).
Participants in the present study were all non-treatment-seeking alcoholics without comorbid psychiatric disorders. We excluded treatment seekers for ethical reasons, and excluded participants with comorbidity to afford the most rigorous test of the hypotheses. Arguably, treatment seekers are the population of interest, and they are likely to have a comorbid mood or anxiety disorder (Grant et al., 2004; Tomasson and Vaglum, 1995; Wu et al., 1999), especially women (Brady et al., 1993). Thus, we can not assume our results generalize to this population.
Despite these limitations, the results of this study are consistent with those from prior studies—alcohol cues can elicit craving, and exposure to an acute psychosocial stressor does not make alcohol cues more potent. Similar to about half of the other clinical laboratory studies conducted in this area, the results did not support that stress increases craving, either generally or cue-elicited, thus suggesting that this assumption, while clinically reported and intuitively appealing, may need to be qualified, and more research is needed to know in whom and under what conditions stress-potentiated craving and cue reactivity occurs.
Table 2.
Demographic, drinking, and psychological characteristics of participants. The only other racial group with substantial representation was African American, comprising 17% of the sample. ADS=Alcohol Dependence Scale; STAI=State Trait Anxiety Inventory; AnxSI=Anxiety Sensitivity Index; BDI=Beck Depression Inventory; BIS=Barrett Impulsivity Scale; DMQ=Drinking Motives Questionnaire; AUQ=Alcohol Urge Questionnaire. Values following each show the min and max scores possible. Men and women did not differ significantly on any of these measures, and there was no gender x stress group interaction (all p’s > 0.05). By chance stress vs. no stress groups differed on percent married, BDI and STAI, which were covaried in outcome analyses.
| No Stress group | Stress group | Effects of stress group, sex, stress x sex |
|||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| 19 | 20 | 21 | 19 | ||
| Demographics | |||||
| Caucasian | 84% | 70% | 86% | 74% | ns |
| Age, M (SD) | 36.5 (13.7) | 38.7 (14.2) | 30.7 (11.9) | 31.9 (11.6) | No stress > stress group, p=.04 |
| % Employed f/t | 26% | 40% | 33% | 47% | ns |
| % Married | 53% | 40% | 10% | 11% | No stress > stress group, p=.01 |
| % Smoker | 37% | 45% | 38% | 26% | ns |
| % College graduate | 47% | 40% | 38% | 47% | ns |
| Alcohol Use, M (SD) | |||||
| Drinks per week | 59.1 (48.8) | 55.1 (34.0) | 51.5 (36.0) | 38.5 (21.8) | ns |
| Drinks per drinking day | 10.2 (7.9) | 94 (5.6) | 10.8 (6.0) | 8.6 (4.7) | ns |
| Alcohol dependence severity, ADS (0–47) | 12.1 (5.4) | 13.6 (6.9) | 12.6 (5.9) | 14.4 (6.9) | ns |
| Age onset alcohol dependence | 23.0 (7.1) | 28.3 (10.9) | 22.9 (51) | 24.6 (7.7) | ns |
| Psychological assessment, M (SD) | |||||
| Trait anxiety, STAI (20–80) | 37.0 (11.1) | 41.7 (9.4) | 33.8 (8.3) | 34.9 (10.0) | No stress > stress group, p= .03 |
| Anxiety sensitivity, AnxSI (0–64) | 15.3 (8.5) | 20.0 (6.9) | 17.2 (7.6) | 17.8 (9.9) | ns |
| Depressive symptoms, BDI (0–63) | 7.9 (8.2) | 12.3 (8.9) | 5.6 (6.5) | 7.6 (8.5) | ns |
| Impulsivity, BIS (30–120) | 68.7 (10.3) | 66.8 (8.5) | 69.1 (11.5) | 64.9 (7.0) | ns |
| Drinks to cope, DMQ (04) | 2.3 (0.7) | 2.7 (0.8) | 2.2 (0.5) | 2.2 (0.6) | ns |
| Alcohol craving, AUQ (8–56) | 18.5 (13.3) | 16.6 (13.3) | 14.3 (11.5) | 20.0 (13.3) | ns |
Acknowledgements
This project was funded by a center grant from NIAAA to the Charleston Alcohol Research Center (P50 AA010761) and by support from the National Center for Research Resources (M01 RR01070).
REFERENCES
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res. 2006;30(6):938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Grice DE, Dustan L, Randall C. Gender differences in substance use disorders. Am J Psychiatry. 1993;150:1707–1711. doi: 10.1176/ajp.150.11.1707. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Stasiewicz PR, Hughes PM, Brimo ML. Trauma-focused imaginal exposure for individuals with comorbid posttraumatic stress disorder and alcohol dependence: revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psych Addict Behav. 2006;20:425–435. doi: 10.1037/0893-164X.20.4.425. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA, Zywiak WH. Male and female alcoholics’ attributions regarding the onset and termination of relapses and the maintenance of abstinence. J Subst Abuse. 1998;10:27–42. doi: 10.1016/s0899-3289(99)80138-2. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LA, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three dimensional measure of drinking motives. Psychol Assess. 1992;4:123–132. [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–275. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment; 1992. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95:129–144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Phillips TS. Alcohol urges in alcohol-dependent drinkers: further validation of the Alcohol Urge Questionnaire in an untreated community clinical population. Addiction. 2002;97:1465–1467. doi: 10.1046/j.1360-0443.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharm. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong K, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319(5869):1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Jansma A, Breteler MH, Schippers GM, de Jong CA, Van Der Staak CF. No effect of negative mood on the alcohol cue reactivity of inpatient alcoholics. Addict Behav. 2000;25:619–624. doi: 10.1016/s0306-4603(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Naber D, Wiedemann K. Hypothalamic-pituitary-adrenocortical axis activity: a target of pharmacological anticraving treatment? Biol Psychiatry. 2006;60:74–76. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test-A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiol. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test—Revisited. In: Harmon-Jones E, Winkielman P, editors. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. New York: Guilford Press; 2007. pp. 56–83. [Google Scholar]
- Lau-Barroco C, Skewes MC, Stasiewicz PR. Gender differences in high-risk situations for drinking: Are they mediated by depressive symptoms? Addict Behav. 2009;34:68–74. doi: 10.1016/j.addbeh.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman GK, Stapleton J, Oppenheim AN, Peleg M, Jackson P. Situations related to alcoholism relapse. Addiction. 1983;78:381–389. doi: 10.1111/j.1360-0443.1983.tb02526.x. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Kadden RM, Gaupp L. Reactivity to alcohol cues and induced moods in alcoholics. Addict Beh. 1990;15:137–146. doi: 10.1016/0306-4603(90)90017-r. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- MacKillop J. Factor structure of the Alcohol Urge Questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol Clin Exp Res. 2006;30:1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology (Berl) 2008;200:141–150. doi: 10.1007/s00213-008-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CG, Brown K. The cue-responsivity phenomenon in dependent drinkers: ‘personality’ vulnerability as intervening variables. Addiction. 1991;86:905–912. doi: 10.1111/j.1360-0443.1991.tb01846.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- National Advisory Council on Alcohol Abuse and Alcoholism. Recommended council guidelines on ethyl alcohol administration in human experimentation. 2005 doi: 10.1111/j.1530-0277.2009.00988.x. Internet url: http://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm (current as of January 2010). [DOI] [PMC free article] [PubMed]
- Nesic J, Duka T. Gender specific effects of a mild social stressor on alcohol cue reactivity in heavy social drinkers. Pharmacol Biochem Behav. 2006;83:239–248. doi: 10.1016/j.pbb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J. Naltrexone decreases craving and alcohol self-administration in alcohol dependent subjects and activates the hypothalamic-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Otto MW, Safren SA, Pollack MH. Internal cue exposure and the treatment of substance use disorders: lessons from the treatment of panic disorder. J Anxiety Disord. 2004;18:69–87. doi: 10.1016/j.janxdis.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Role of the HPA axis and the A118G polymorphism of the mu-opioid receptor in stress-induced drinking behavior. Alcohol Alcohol. 2009;44(4):358–365. doi: 10.1093/alcalc/agp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Abrams DB, Rubonis AV, Niaura RS, Sirota AD, Colby S. Cue elicited urge to drink and salivation in alcoholics: Relationship to individual differences. Adv Behav Res Ther. 1992;14:195–210. [Google Scholar]
- Rosenberg H. Clinical and laboratory assessment of the subjective experience of drug craving. Clin Psychol Rev. 2009;29:519–534. doi: 10.1016/j.cpr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 1994;55:487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Rubin A, Stout RL, Longabaugh R. Gender differences in relapse situations. Addiction. 1996;91:S111–S120. [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Baker R, Harnett-Sheehan K, Knapp E, Sheehan M. MINI International Neuropsychiatric Interview, English version 5.0, DSM-IV. Tampa: University of South Florida Press; 2002. [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2008;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist KL, Bhagwager Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiologic responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychological and biological methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Stasiewicz PR, Gulliver SB, Bradizza CM, Rohsenow DJ, Torris R, Monti PM. Exposure to negative emotional cues and alcohol cue reactivity with alcoholics: a preliminary investigation. Behav Res Ther. 1997;35:1143–1149. [PubMed] [Google Scholar]
- Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. J Subst Abuse. 1997;9:223–240. doi: 10.1016/s0899-3289(97)90018-3. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) Br J Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: The role of automatic and non-automatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tómasson K, Vaglum P. A nationwide representative sample of treatment-seeking alcoholics: a study of psychiatric comorbidity. Acta Psychiatrica Scandinavica. 1995;92:378–385. doi: 10.1111/j.1600-0447.1995.tb09600.x. [DOI] [PubMed] [Google Scholar]
- van den Bos R, Harteveld M, Stoop H. Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology. 2009;34:1449–1458. doi: 10.1016/j.psyneuen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Wu LT, Kouzis A, Leaf PJ. Influence of comorbid alcohol and psychiatric disorders on utilization of mental health services in the National Comorbidity Survey. Am J Psychiatry. 1999;156:1230–1236. doi: 10.1176/ajp.156.8.1230. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Westerberg VS, Connors GJ, Maisto SA. Exploratory findings from the Reasons for Drinking Questionnaire. J Sub Abuse Treatment. 2003;25:287–292. doi: 10.1016/s0740-5472(03)00118-1. [DOI] [PubMed] [Google Scholar]