Figure 2. Glutathione metabolism in alcoholic skeletal muscle.
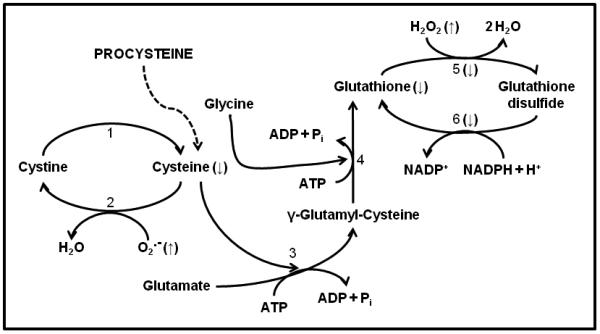
Alcoholic myopathy is characterized by increased formation of superoxide (O2.−), lipid and hydrogen peroxide (H2O2), and reactive oxygen species. Glutathione cycling is also altered suggesting a reduced capacity to sequester these alcohol-induced oxidants. Supplementing the diets of alcoholics with L-2-oxothiazolidine-4-carboxylate, a proform of L-cysteine (Procysteine), normalizes thiol levels of cysteine and glutathione. Enzymes that catalyze these reactions are: (1) glutathione dependent thiodisulfide, thioltransferase, and non-enzymatic reactions; (2) protein disulfide isomerase; (3) γ-glutamylcysteine synthetase; (4) glutathione synthetase; (5) glutathione peroxidase; and (6) glutathione reductase. Arrows (↑, ↓) denote direction of change as a result of chronic alcohol abuse. Other abbreviations used: ADP, adenosine diphosphate; ATP, adenosine triphosphate; H+, hydrogen ion; H2O, water; NADP+, nicotinamide adenine dinucleotide phosphate, NADPH, reduced form of NADP+; Pi, inorganic phosphate.
