Abstract
Primary infection by varicella zoster virus (VZV) typically results in childhood chickenpox, at which time latency is established in the neurons of the cranial nerve, dorsal root and autonomic ganglia along the entire neuraxis. During latency, the histone-associated virus genome assumes a circular episomal configuration from which transcription is epigenetically regulated. The lack of an animal model in which VZV latency and reactivation can be studied, along with the difficulty in obtaining high-titer cell-free virus, has limited much of our understanding of VZV latency to descriptive studies of ganglia removed at autopsy and analogy to HSV-1, the prototype alphaherpesvirus. However, the lack of miRNA, detectable latency-associated transcript and T-cell surveillance during VZV latency highlight basic differences between the two neurotropic herpesviruses. This article focuses on VZV latency: establishment, maintenance and reactivation. Comparisons are made with HSV-1, with specific attention to differences that make these viruses unique human pathogens.
Keywords: herpesvirus, human ganglia, latency, Varicella zoster virus, VZV
A central feature common to all members of the Herpesviridae family is their ability to establish latent infection in their natural host. Varicella zoster virus (VZV), a member of the alphaherpesvirus subfamily, shares similar biologic features and genomic organization with herpes simplex virus type 1 (HSV-1), the prototype neurotrophic human alphaherpesvirus. Both viruses are typically acquired early in life, during which time both viruses gain access to ganglia where they become latent. Reactivation from latency results in replication and shedding of infectious virus, which ensures the virus is transmitted to a naive population [1]. While the theme is similar, sufficient nuances exist to warrant classification of HSV-1 and VZV into separate subfamilies (Simplexvirus and Varicellovirus, respectively). This article focuses on the latent state of VZV with reference to HSV-1 for comparison and clarification.
VZV latency in the postvaccine era
In the prevaccine era, varicella (chickenpox) was a common childhood disease, peaking in late winter to early spring. Most cases occurred within the first decade of life, resulting in essentially universal exposure (>90% VZV seroconversion) by the age of 17 years [2,3]. Following varicella vaccine licensure in 1995, hospital complications associated with varicella declined by three-quarters with an annual saving of US$85 million [4]. Although time-dependent loss of VZV immunity following vaccination has altered the Centres for Disease Control and Prevention recommendations to include a second vaccine dose [5], the vaccine is remarkably safe [6], and vaccination, not herd immunity, is successful in protecting a majority of the child population [7]. However, the varicella vaccine, which is an attenuated clinical isolate [8], is a mixed population of mutated viruses [9]. Thus, a movement is underway to construct a new vaccine, based on a pure strain of attenuated virus [10] or specific VZV proteins [11].
In addition to a vaccine to protect against primary infection (chickenpox), a vaccine has also been licensed to protect against zoster (shingles), the major clinical outcome of VZV reactivation. This vaccine is also based on the attenuated virus, albeit in a formulation containing higher virus titer. The shingles vaccine reduced the burden of illness by 61.1% in a cohort of more than 25,000 elderly individuals during an approximately 3-year (median) surveillance period [12]. However, the varicella vaccine is associated with breakthrough disease, even in highly vaccinated populations [13], and the attenuated virus can establish latency and reactivate to cause zoster in a similar way to the wild-type virus [14].
The shingles vaccine is aggressively advertised; however, universal vaccination is doubtful owing to vaccine cost, health insurance reimbursement policies [15], lack of education and vaccine storage requirements [16]. In addition, the vaccine protects only half of individuals older than 60 and only 38% of individuals over 70 years of age [17]. Thus, even if universal coverage was obtained, at least 500,000 of the approximately 1 million new zoster cases per year would still occur [18,19]. Therefore, even in this postvaccine era, VZV latency remains a concern, especially for the elderly and immunocompromised.
From primary infection to the ganglion
Varicella zoster virus, like HSV-1, is readily transmitted in aerosol droplets or through direct contact with secretions or broken vesicles. Primary infection by both alphaherpesviruses results in virus replication, during which time the virus gains access to ganglionic neurons and establishes latency. While primary HSV-1 infection is typically mild and frequently goes unnoticed, the fever, malaise, headache, abdominal pain, anorexia, occasional vomiting, and cutaneous vesicular, ulcerative, pruritic (itchy) and often painful lesions on an erythematous base, lasting 10–21 days that accompany primary VZV infection are more noticeable [20,21]. Varicella in otherwise healthy children is typically benign, but can cause death in approximately 0.06% of the total varicella cases and approximately 20% of varicella cases involving the CNS [22]. Widespread (dual dose) vaccination has diminished the frequency of varicella while simultaneously making diagnosing primary VZV infection challenging [23]. As more individuals become vaccinated, understanding the molecular biology and population dynamics of the attenuated virus will become increasingly important; however, current studies have focused on wild-type virus. Consequently, this article will highlight the wild-type virus.
Following exposure, VZV infects mucosal dendritic cells of the nasal pharyngeal region, along with Langerhans and plasmacytoid dendritic cells in the respiratory mucosa [24,25]. VZV-infected dendritic cells migrate to draining lymph nodes where resident T cells become infected and express cutaneous lymphocyte-associated antigen along with chemokine receptor type 4 [26]. The infected CD4+ T cells expressing skin homing factors transit to dermal endothelial cells, where the virus infects dermal fibroblasts and keratinocytes [27]. As the infection proceeds, cytokine-induced inflammation results in the formation of the characteristic varicella rash [28]. Within vesicular fluid, cell-free virus accumulates in sufficient amounts to distinguish vaccine from wild-type VZV as well to genotype the virus into one of the existing five circulating clades thus far identified [29–32].
This cell-free stage of the virus life cycle is unique. When grown in cell culture, the virus is highly cell associated, with a particle:PFU ratio of approximately 40,000:1 [33]. Most likely, a large percentage of maturing virus is targeted to late endosomes for degradation [34]. The degradation pathway involves recognition of mannose-rich VZV glycoprotein intermediates by the cellular mannose 6-phosphate receptor present in most cell types. Since the mannose 6-phosphate receptor protein is downregulated during dermal cell terminal differentiation, targeting of VZV glycoproteins for degradation is reduced and release of cell-free virus becomes increasingly efficient as infection percolates to the skin surface. Sensory nerves of the dorsal root terminate within the dermis and are located at the region where infectious VZV is released. Thus, axonal infection by cell-free VZV or by cell-associated virus through virus-induced filopodia is likely. Both HSV-1 and VZV induce filopodia formation, which is used by the virus to travel towards the cell body, but is also available to propell the virus into neighbouring cells [33,35,36]. Thus, VZV, like HSV-1, may enter neuron termini, associate with the cell’s retrograde transport machinery and transit to the nucleus [37].
Since varicella lesions can be present on all areas of the body, VZV has potential access to, and the ability to reactivate from, ganglia over the entire neuraxis. The classic tome describing zoster skin lesions confirmed this hypothesis long before the etiology was known [38]. This hypothesis was confirmed in the current era of molecular biology, through PCR performed on DNA extracted from human ganglia removed at autopsy. VZV DNA has been found in geniculate, vestibular, trigeminal, cervical, thoracic and sacral ganglia [39–44]. Thus, VZV can establish latent infection in ganglia from all regions of the body, unlike HSV-1, which is generally restricted to ganglia of the head and neck. HSV-1 DNA is present in geniculate, vestibular, olfactory and multiple cranial (especially trigeminal) ganglia, but only rarely in dorsal root ganglia [44–48].
Seeding the ganglion with virus
Classic studies involving infecting mouse foot pads with herpes virus followed by paw amputation at various times postinfection suggest that, along with direct neuronal transit, the virus may access distal neurons via a hematogenous route [49]. The finding that genotypically different VZV isolates were recovered from the same 30-year-old individual during two episodes of zoster, despite only one reported varicella experience, suggests multiple ganglionic seedings, one from varicella and a second from a hematogenous route from subclinical exposure [50]. The possible hematogenous route of ganglionic infection by alphaherpesviruses is confirmed by the detection of simian varicella virus, the monkey counterpart of human VZV, DNA in trigeminal ganglia (TG) prior to the development of the characteristic cutaneous lesions [51]. However, most investigations concerning ganglionic infection focus on transit of virus to the neuronal nucleus following infection at axonal termini.
Under the scenario of centripetal transit of virus from skin, the first question to address concerns the requirement for virus replication in the skin prior to axonal infection. This question has been addressed using the mouse and rabbit models of HSV-1 latency [52,53]. The first set of experiments used HSV-1 containing a mutation in the virus thymidine kinase (Tk) gene. In the mouse model, Tk activity and virus replication in the eye is required for HSV-1 to infect the TG [54]. However, in the rabbit model, Tk activity is not required for ganglionic infection [55]. Further studies using the mouse model demonstrate that only minimal Tk activity is sufficient for ganglionic infection [56] and that HSV-1 Tk-null virus can replicate in neuronal cells [57]. In addition, using PCR to increase the detection of virus DNA, low copies of HSV-1 Tk-null DNA were found in mouse TG following corneal infection [58]. These experiments show that peripheral Tk activity is not essential, but does increase latent virus burden in TG, and highlight the difference between the mouse and rabbit models, a theme that has continued to date [59].
Herpesvirus replication involves the exquisite orchestration of virus genes whose products have been classified into groups dependent upon the timing of their transcription [60]. The universality of this classification is demonstrated by the fact that the three major classes genes initially identified [61] are still a major characteristic used to describe the kinetics of herpes-virus infection [62]. Alpha or immediate-early (IE) proteins are the first virus genes synthesized postinfection. As IE proteins are generally present in the virion tegument and can initiate transcription from their own promoters, they require no prior cell protein synthesis, and are generally involved in reprogramming the host transcriptional machinery to recognize virus promoters. Beta or early (E) proteins are the second temporal class of virus genes expressed, require IE protein for efficient transcription and are generally proteins involved in DNA replication. Gamma or late (L) proteins are transcribed following initiation of virus DNA replication, and are generally structural proteins.
Herpesvirus Tk is an E protein, the presence of which signifies that extensive virus gene expression has already transpired. To more clearly define the relationship between virus replication and ganglionic infection, HSV-1 mutations were constructed to ablate selected IE protein activity. When scarified mouse corneas were infected with HSV-1 containing mutations in essential IE proteins (ICP27 or ICP4), no virus DNA was detected in TG by DNA hybridization [63]. However, using PCR, HSV-1 DNA was detected in mouse TG infected with the IE-null HSV-1 [58]. Thus, again, we see that while virus replication is not a strict requirement to seed ganglia, peripheral virus replication increases the viral burden within the ganglion.
Can the same be said for VZV? Before this question can be addressed, two requirements must be met: an animal model must be developed and mutations in essential VZV genes must be constructed. The small animal model of VZV latency used most often is the rat [64]. VZV-infected cells are inoculated subcutaneously along the spine and animals are maintained for up to 9 months. Within this model, no primary infection is seen, the virus cannot be reactivated and while the virus is not present in lung, liver or kidney, it is infrequently present in spleen [65]. Thus, rats may provide a model for VZV ganglionic infection; however, its application to latency is still debated. Nonetheless, the rat model can be used to address the requirement for virus replication prior to axonal seeding.
Insertion of defined mutations into the VZV genome is problematic. The traditional method to insert defined mutations into the genome of alphaherpesviruses involves PCR construction of target DNA, in vivo recombination into virus-infected cells and plaque purification of the mutated virus [66]. While PCR technology readily permits construction of DNA fragments with defined mutations in selected VZV genes, and in vivo recombination will insert the large recombinant DNA fragment into the virus genome, the lack of cell-free virus hinders VZV plaque purification. Consequently, isolation of defined mutations requires multiple rounds of virus DNA extraction, transfection and propagation of selected foci of infected cells followed by extensive DNA analysis to ensure genetic purity of the virus. To solve this problem, the VZV genome has been dispersed among a set of overlapping cosmids [67,68] or inserted into a plasmid for propagation as a bacterial artificial chromosome [69–73]. Insertions of defined mutations, either by traditional restriction endonuclease technology or through induction of the host bacterial recombination system, has permitted selection of VZV containing defined genetic mutations with relative ease. Thus, both the technology and model system is available to broach the question of the requirement for VZV replication prior to ganglionic infection.
Can the rat model of ganglionic infection with specific gene mutations in VZV be used to address the need for virus replication prior to ganglionic infection? VZV Tk is a dispensable gene for virus replication in tissue culture [67], and not a desirable candidate gene to test. Similarly, VZV genes encoding the large subunit of ribonucleotide reductase (open reading frame [ORF] 19; [74]), glycoprotein C (ORF 14; [75]), transactivators of gene transcription (ORF 10, [76]; ORF 61, [77]) or a membrane protein unique to VZV (ORF 1; [78]) yield infectious virus and are also not suitable to test. However, three VZV genes have been tested for their ability to influence ganglionic infection. Deletion of VZV ORFs 4, 29 and 63 yields virus that seed rat ganglion to significantly reduced infection levels compared with wild-type virus [79,80–82].
Varicella zoster virus ORFs 4 and 29 are required for virus growth in tissue culture [80]. VZV ORF 63 is dispensable for virus growth in malignant melanoma cells [83] but is required for efficient virus growth in skin organ cultures [84]. Thus, the rat model of VZV latency has aided our understanding of the virus proteins required to infect ganglia and maintain virus DNA within neuronal cells over time [85]. In summary, primary VZV replication at the site of inoculation increases virus burden in the ganglion in a similar way to HSV-1. However, since HSV-1 and VZV DNA are detected in ganglia in the absence of detectable virus replication, seeding ganglia may also occur through a passive (independent of virus replication) event.
Virus in the neuron
Under the scenario of centripetal virus transit, the second question to address is regarding the cell type and virus burden during latency. If ganglia become seeded with virus through retrograde transport from peripheral lesions, the neuron would be the predicted site of virus latency. Initial in situ hybridization showed that both HSV-1 and VZV nucleic acid localized to neurons in latently infected human TG [86]. The frequency of HSV-1 detection did not change with DNase treatment but diminished with RNase treatment, indicating that the signal detected virus transcripts. The VZV signal could originate from either nucleic acid since nuclease treatment was not performed on these sections. The HSV-1 results became clear when an abundant virus transcript, the latency-associated transcript, was detected exclusively in neurons in TG from latently infected rabbits [87], mice [88] and humans [89]. However, the VZV results took a different path when it was reported that VZV RNA, unlike HSV-1 RNA, localized to satellite cells surrounding neurons in latently infected human TG [90].
The finding that HSV-1 and VZV latency was maintained in different cell types made perfect sense from a clinical standpoint [91]. HSV-1 reactivation typically causes highly localized skin lesions (cold sore) whereas VZV reactivation typically encompasses one to three dermatomes (shingles). The hypothesis was that when HSV-1 reactivated, the virus would track to the skin through the few latently infected neurons, while VZV reactivation in satellite cells would infect multiple neurons and hence cause a widespread rash. However, from a molecular approach, this made little sense. VZV and HSV-1 are very similar neurotropic alphaherpesvirus. Although at the molecular level, homologous proteins do not necessarily possess identical function [92], the differences in genomic content do not explain the large functional differences required to maintain latency in different cell types: nondividing neurons versus replicating satellite cells. The VZV question was eventually answered by in situ DNA-PCR amplification [93], analysis of neurons isolated from dissociated TG [94,95] and, finally, laser-capture microdissection [96], which demonstrated, through the use of multiple different approaches, that VZV, like HSV-1, resides in ganglionic neurons during latency.
Since both the number of HSV-1-infected neurons [97] and the virus DNA burden per neuronal cell [98] affect HSV-1 reaction frequency in the mouse model, effort has been made to determine the VZV DNA burden during latency. By necessity, the analysis of VZV latency requires the study of human ganglia removed at autopsy. The study of human autopsy tissue is fraught with concerns: first, only prospective/description studies can be performed; second, samples are collected from outbreeding populations; third, samples are collected from individuals infected with different virus strains; fourth, samples are obtained at varying times postmortem; and finally, samples are collected from individuals with diverse health histories and whose deaths follow extremely varied courses, from acute (e.g., automobile accidents) to protracted (e.g., coronary disease). Taking into account these caveats, the single most important piece of information concerning a particular report is sample size. Table 1 summarizes the studies in which HSV-1 or VZV DNA were detected in human TG. The prevalence of HSV-1 ranges from 53 to 100% of TG analyzed, with an average virus burden ranging from 111 to 2902 copies per TG. Similarly, the prevalence of VZV ranges from 63 to 100% of TG analyzed with an average virus burden ranging from 258 to 1798 copies per TG. Reviewing the report with the largest sample size indicates that VZV is approximately 1.5-times more prevalent than HSV-1 [99]. Normalizing the quantitative results to reflect the number of virus DNA copies per 105 cells indicates that the burden for both viruses ranges from hundreds to thousands of copies per TG. The number of virus DNA copies per individual neuronal cell is low but similar between VZV and HSV-1: 11.3 copies of HSV-1 DNA per neuron and 6.9 copies of VZV DNA per neuron [96]. Thus, the clinical differences between HSV-1 and VZV reactivation cannot be explained by fundamental differences in the cell type, virus burden per ganglion or virus burden per cell in latently infected TG.
Table 1.
Herpes simplex virus type 1 and Varicella zoster virus DNA in human trigeminal ganglia.
| Sample size | Positive ganglion (%)
|
Virus DNA copy number
|
Ref. | ||
|---|---|---|---|---|---|
| HSV-1 | VZV | HSV-1 | VZV | ||
| 10 | 100 | 100 | NR | NR | [42] |
|
| |||||
| 11 | 73 | 91 | NR | NR | [43] |
|
| |||||
| 12 | NR | NR | 111 | NR | [182] |
|
| |||||
| 14 | NR | 79 | NR | NR | [39] |
|
| |||||
| 15 | 53 | 87 | 2902 | 258 | [183] |
|
| |||||
| 17 | 70 | 100 | 1777 | 8990 | [130] |
|
| |||||
| 17 | 94 | 88 | NR | NR | [39] |
|
| |||||
| 109 | 73 | 63 | NR | NR | [46] |
|
| |||||
| 174 | 90 | NR | 580 | NR | [184] |
|
| |||||
| 414 | 61 | 94 | NR | NR | [99] |
HSV-1: Herpes simplex virus type 1; NR: Not reported; VZV: Varicella zoster virus.
Establishing latent infection in the neuron
Currently, little information is available concerning the molecular events by which the VZV genome is silenced in the neuronal nucleus resulting in latency. In productively infected cells, HSV-1 DNA replication results in a diverse population of molecules ranging from unit-length, double-stranded, linear or circular genomes to large replicative intermediates consisting of multiple genomes looped and tangled together [100–103]. These structures resolve during latency and the virus genome is present in circular, episomal form in the neuron [104,105]. Early stages of HSV-1 DNA replication take place near a complex of host proteins consisting of promyelocytic leukemia protein (PML), speckled protein of 100 kDa (SP100) and a death-domain-associate protein (Daxx) [106,107]. These PML-containing nuclear bodies are disrupted early in HSV-1 infection by HSV-1, ICP0, a virus-encoded IE protein. An excellent review of HSV-1/PML interaction recently appeared in an earlier edition of Future Virology [108]. Briefly, the balance between ND-10-induced HSV-1 gene silencing and HSV-1 ICP0-mediated ND-10 degradation influences the outcome of virus infection. ICP0-dependent ND-10 degradation is a major contributing factor to continued HSV-1 gene expression and productive (lytic) infection [109], and the silencing of HSV-1 gene transcription by ND-10 action is a major component in the establishment of quiescent (latent) infection [110,111]. While excellent correlation between ND-10 function and the establishment of latency in tissue culture exists, the involvement of ND-10 structures in the silencing of HSV-1 gene expression in animal models of latency has not yet been demonstrated.
The VZV protein encoded by ORF 61 (61p) is a HSV-1 ICP0 homolog. VZV 61p also induces dismantling of host ND-10 structures, which facilitates virus replication [112]; however, VZV 61p does so by degrading SP100 [113]. While ND-10-induced silencing of VZV gene expression is an attractive mechanism, significant work remains to be done. Even so, the end result is similar; during latency, both VZV and HSV-1 genomes are predominately silent, and in an endless, circular, most probably single-unit, episomal form [114].
Maintaining latency in the neuron
Analysis of numerous autopsy samples collected from individuals of varying age suggests latency is established upon primary infection, and is maintained for life [99]. Evidence from the mouse model of HSV-1 latency suggests limited and selected neuronal cell death during the transition from acute to latent infections [115]. This cell death may take on the form of apoptosis [116], thus, it is beneficial, if not essential, for the virus to block apoptosis during latency. HSV-1 gene transcription is restricted to a single locus in latently infected neurons. While reports of a HSV-1 protein expressed during latency exist [117,118], latency-associated transcript (LAT) functions, in part, at the RNA level [119] by an unknown mechanism to inhibit apoptosis [120]. However, HSV-1 LAT, a stable intron [121,122] spliced from an unstable 8.3-kb primary transcript [123], maps to a location on the HSV-1 genome that is not present in VZV DNA [124].
A study of 1718 patients with suspicious skin lesions revealed both VZV and HSV-1 in 1.2% of the cases, indicating that both neurotropic alphaherpesviruses can reactivate at the same time [125]. Since VZV and HSV-1 can become latent in the same ganglion [126] and in the same neuronal cell [127], one would expect VZV, like HSV-1, to encode an antiapoptotic function. The search for VZV genes expressed during latency has used in situ hybridization [40,128,129] and in situ immunohistochemistry [42,130–134]. However, in situ technology is capricious and prone to investigator interpretation [135]. Thus, the most reliable data comes from cDNA cloning and sequence analysis demonstrating a typical mRNA structure including a downstream poly(A) tail. Using this more stringent criterion, transcripts mapping to VZV ORF 21, 29, 62, 63 and 66 have been detected in latently infected human ganglia [136–138]. Quantitative analyses demonstrate that VZV ORF 63 is the most abundant and prevalent of the latently transcribed genes [130,139]. Since the protein encoded by VZV ORF 63 (VZV ORF 63p) is present in latently infected neurons [133], VZV ORF 63p is the most likely candidate VZV gene expressed during latency to contain an anti-apoptotic function. Therefore, it was gratifying when VZV ORF 63p was shown to inhibit apoptosis in human ganglia infected in vitro [140]. Care must be exercised so that the results are not oversimplified. For example, a recent study suggests that VZV ORF 63p expression is exceedingly rare [141], which highlights the fact that much work is yet to be done in the area of VZV gene expression during latency. However, an interesting start has been made.
Reactivation from latency
Immunosenescence is a relatively new field that has experienced exponential growth in the past decade. Immunosenescence is the natural decline in T-cell function with age [142,143]. While its cause is unknown, chronic antigen stimulation resulting from frequent asymptomatic herpesvirus (cytomegalovirus) reactivation has been implicated [144,145]. Advancing age with the accompanying decline in VZV responder T-cell frequency is the single most important factor influencing VZV reactivation in otherwise healthy individuals [146,147]. Taking T-cell depletion to an extreme, the incidence of zoster is greater than tenfold higher in HIV seropositive individuals than in age-matched controls [148], suggesting that the occurrence of VZV reactivation follows a full spectrum of immunosuppression, from natural decline associated with immunosenes-cence to disease-induced immunosuppression or immunosuppressive therapy [149]. Even transient stress-induced immunosuppression [150] or trauma [151] can reactivate the VZV. Thus, the most likely link between a waning immune system and zoster is a lack of virus clearance during episodes of virus reactivation.
Reactivation of latent HSV-1 in TG can be seen as a two-step process involving initiation of virus gene expression followed by virus replication leading to the production and release of progeny virions [152]. The stress-associated host factors that alter the nuclear environment in latently infected neurons are poorly characterized, but most likely involve altering the epigenetic markings on the latent virus genome [59,153,154], resulting in de novo synthesis of VP16 [155]. VP16, along with host proteins, form a complex at specific sites on the HSV-1 genome, thereby activating IE gene transcription [156,157], and initiating the cascade of events, which, if unchecked, culminate in the release of newly synthesized virus.
During virus replication, the newly synthesized proteins are potential targets for immune clearance by sentinel CD8 memory cells. This is a potential checkpoint between initiation of reactivation and release of infectious virions.
At autopsy, neurons containing latent HSV-1 are frequently surrounded by a corona of CD8 T cells expressing markers of antigen activation [134,158]. The granzyme B-containing immune cells recognize HSV-1 proteins synthesized during virus reactivation, fuse to the neuron and release their cargo [159,160], but apoptosis is not induced. It is thought that granzyme B-induced apoptosis is blocked in the latently infected neurons by LAT’s antiapoptotic function [161]. Virus reactivation may be inhibited by granzyme B-dependent cleavage of HSV-1 ICP4, the major virus IE protein involved in gene activation and required for virus replication [159]. Since the immunodominant epitope recognized by resident CD8 T cells in latently infected ganglia is a linear eight amino acid region in the center of glycoprotein B (gB) [162] a late virus protein, the synthesis of which is dependent on functional ICP4 timing is critical. For example, how can sentinel CD8 T cells recognize gB and degrade ICP4 to block virus reactivation when functional ICP4 is required for gB synthesis? Recent work points to a resolution. While approximately half of the resident CD8 T cells that respond to HSV-1 recognize gB, there is a pool of cells that do not recognize this late glycoprotein [163]. It is possible that gB-nonresponsive CD8 T cells recognize virus proteins presented earlier during the reactivation process and thereby quell reactivation prior to HSV-1 late protein synthesis.
Immune surveillance and T-cell-dependent proteolysis of critical viral proteins may check HSV-1 reactivation, but the same does not appear to be true in VZV. Analysis of human TG indicates a lack of T cells surrounding neurons containing latent VZV [158,160]. The lack of VZV-specific immune surveillance during latency cannot be attributed to the lack of virus proteins synthesized during latency. VZV transcripts [130,139] and, more importantly, proteins encoded by ORFs 21, 29, 62, 63 and 66 [131,133,164] are present in the cytoplasm of latently infected human TG. It is possible that major histocompatibility complex (MHC) presentation is blocked. Minimal heavy chain class 1 MHC is expressed in neurons [165] and VZV ORF 66 protein, a gene expressed during latency [138], down-regulates MHC class 1 expression [166]. Thus, the lack of CD8 T cells in TG latently infected with VZV may result from a virus-specific mechanism to inhibit MHC presentation coupled with the naturally low expression of MHC on neurons.
Analysis of TG from subjects who had experienced zoster 1–4.5 months prior to death (unrelated to VZV) shows an increase in CD3+ T cells (prominently CD8+, granzyme B negative), CD20 (B cells) and CD57 (natural killer cells) [167]. These precious samples are seldom obtained and offer a unique opportunity to study the natural processes during recovery from zoster. To investigate the signal that recruits T cells to the site of VZV reactivation, even rarer ganglia were analyzed [168]. Human dorsal root ganglia at the L2 dermatome were removed from a 92-year-old individual who had L2-distributed zoster at the time of death (unrelated to zoster). In situ analysis revealed VZV glycoprotein E expression, indicating virus reactivation/replication, and extensive neuronal expression of CXCL 10. Thus, the immune system is active in clearing virus during reactivation by chemokine-dependent T-cell recruitment, but not necessarily required to keep virus in check during latency.
Clinical outcome of VZV reactivation
In immunocompetent individuals, VZV reactivation (shingles) is typically a single event, unlike HSV-1 where multiple and frequent reactivations (cold sores) are often seen. Typically, cold sores are painful, but small and localized, and cosmetically distressing, but resolve within 2 weeks. However, shingles is characterized by severe, sharp, lancinating, radicular pain and a vesicular eruption on an erythematous base, involving one to three dermatomes with residual pain and scarring lasting for many months. Zoster has well earned its Norwegian moniker: ‘a belt of roses from Hell’ [169]. The most frequent and debilitating complication of zoster is neuropathic pain persisting beyond the duration of rash. Pain that lasts more than 3 months following rash is known as postherpetic neuralgia. In addition, VZV reactivation can cause vasculopathy of large or small cerebral arteries [170], leading to transient ischemic attacks [171] and stroke [172]. VZV-induced stroke is especially insidious, since its association with the elderly is often overlooked [173]. VZV is the only herpesvirus directly associated with stroke [172], and this potentially fatal event [174] is treatable. VZV reactivation can lead to Ramsay Hunt syndrome, with virus DNA and proteins detectable in the middle ear [175]. VZV reactivation can also cause disease of the spinal cord, acute or progressive outer retinal necrosis [173] and zoster sine herpete or dermatomal pain in the absence of rash [149]; however, the full clinical descriptions are beyond the scope of this article.
Conclusion
Varicella zoster virus, one of the three human alphaherpesviruses, causes serious neurologic disease upon reactivation. Despite vaccines against varicella and zoster, virus reactivation remains a significant concern, especially among elderly and immunocompromised individuals. The lack of a suitable animal model of VZV pathogenesis and latency requires analysis of clinical and autopsy samples as well as detecting parallels with HSV-1. Analysis of autopsy samples has shown that, while both the latent HSV-1 and VZV genome are circular and associated with histones, control of VZV gene transcription is less stringent. In addition, while inhibition of neuronal apoptosis may be a feature common to alphaherpesviruses, the mechanism by which this is done may differ between HSV-1 and VZV. Figure 1 shows a major difference between the HSV-1 and VZV genomes. The approximately 9.1-kbp unique long repeat in HSV-1 DNA is truncated to approximately 88 bp in VZV. Since HSV-1 LAT and the major virus-encoded miRNA map to this repeat, VZV has no LAT ortholog and encodes no miRNA [176]. It is possible that LAT’s antiapoptotic function may be supplied by VZV IE63. Other VZV transcripts that have been detected at lower and more inconsistent frequencies may reflect their gene location with respect to epigenetic markings on the latent virus genome. While an answer to this question may be supplied through descriptive analysis of multiple latently infected ganglia, the mechanism by which VZV reactivates must await the development of a means to perform prospective experiments on latently infected human ganglia. To this end, Zerboni et al. (2005) have developed an exquisite means by which human dorsal root ganglia are maintained for extended times as xenografts in severe combined immuno-deficient mice [177,178]. Although this model lacks a functional immune system, it has been instrumental in demonstrating that human ganglia can be infected via a hematogenous route through circulating T cells, and that the infected ganglia progress to a latent state with the transcription of VZV gene 63 [177]. The ganglion xenografts are also a promising system to assess the function of specific VZV proteins in virus neurovirulence [179]. In addition, human ganglionic xenografts may shed light on the clinical differences between VZV and HSV-1 reactivation. VZV-infected neurons show significant fusion to surrounding satellite cells [180]. These virus-induced neuron–satellite syncytia may result in multiple neurons becoming infected through the VZV reactivation within a few isolated neurons, thus causing the ganglionic distribution observed in zoster. Future advances in the analysis of VZV latency will depend on this and other novel models that reflect the natural features of this uniquely human neurotropic alphaherpesvirus.
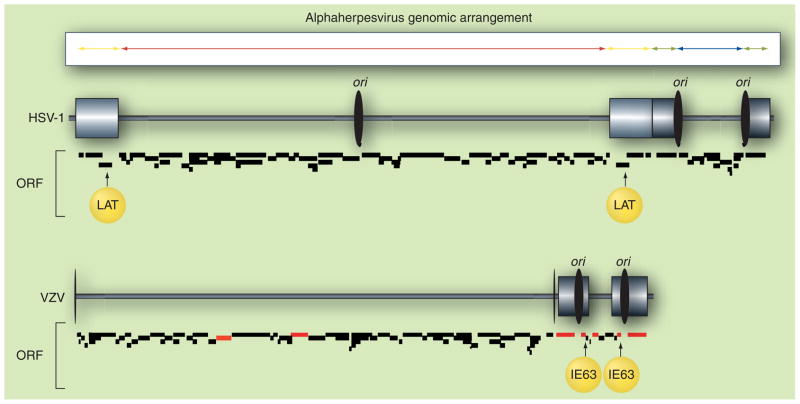
Figure 1. Herpes simplex virus type 1 and varicella zoster virus genomic structures.
The alphaherpesvirus genome is composed of two unique stretches of DNA: unique long (red) and unique short (blue). Each unique segment is covalently joined through smaller regions of inverted repeats: repeat of unique long (yellow) and repeats of unique short (green). The HSV-1 genome preserves the basic structure even though the unique regions with their attendant repeats are inverted, resulting in four isomeric forms of the virus DNA. The VZV genome lacks approximately 9 kb of DNA from each unique long repeat, which may explain why this region rarely (5%) inverts with respect to the unique short region [181]. More importantly, the unique long repeat region in HSV-1 contains the LAT locus, the major virus transcript present during latency. VZV IE63, immediate–early protein encoded by ORF 63, may fulfill some of LAT’s functions, since like LAT, ORF 63 is the most abundant and prevalent virus transcript detected during latency, and since both LAT and IE63 block virus-induced apoptosis. The red ORF in the VZV genome indicates genes whose transcripts have been cloned and sequenced from latently infected human ganglia. The ORF annotations of HSV-1 (accession number NC_001806) and VZV (accession number NC_001348) genome were supplied by PubMed. HSV-1: Herpes simplex virus type 1; LAT: Latency-associated transcript; ori: Origin of DNA replication; ORF: Open reading frame; VZV: Varicella zoster virus.
Future perspective
In many aspects, this is the golden age of VZV research. Armed with the complete genome of many clinical isolates, the geographic distribution of the virus is becoming evident. Increasingly, it is evident that this virus is very stable and the identified clades are more for tracking purposes than indicators of disease outcomes. We also have tools sensitive enough to detect virus footprints at levels approaching one VZV genome per 400 cells and one VZV transcript in a pool of 3.5 × 108 averaged-sized mRNA. High-throughput technology has also been applied to VZV and arrays are available for rapid genotype identification, along with analysis of all predicted virus transcripts. Once interesting VZV genes have been identified, BAC-directed mutagenesis is available to construct site-directed mutations in specific genes or knockouts in specific transcription factor-binding sites. In addition, large-scale, nonbiased analyses of the virus have been performed to identify all VZV protein–protein interactions as well as to identify all VZV genes essential for virus replication in cell culture.
Nonetheless, major areas in VZV research still lag behind similar studies in HSV-1. VZV latency research is stymied due to the lack of a small animal model in which virus latency can be established and experimentally reactivated. In addition, we do not know the extent of disease produced by VZV reactivation. Future efforts are required to expand the scope of our knowledge concerning disease produced by VZV reactivation (clinical research) and the mechanism of virus reactivation (basic research).
In addition, the basic biology of VZV in cell culture is meager compared with what we know concerning the lytic life cycle of HSV-1. The lack of cell-free virus is a major contributing factor for this deficit. We may know why VZV is not released from infected cells in culture, but we have not yet put that knowledge to use. Infections are still performed by cocultivations and provide a view of the virus as it advances through the culture. We do not know the events transpiring in a one-cycle virus growth period.
Finally, the vaccine has been a great benefit to the human population. Deaths associated with primary varicella have been decimated and regarding zoster, the major outcome of VZV reactivation, deaths have been reduced by two-thirds. The long-term outcomes of vaccination are not yet known. Will there be a change in the demographic of VZV infections? There is evidence that primary VZV infection presents differently than classic chickenpox and may be missed by parents and pediatricians. Will virus reactivation also present differently? Will we notice a different set of diseases due to VZV reactivation? The current live vaccine, which is composed of genetically different viruses, must be analyzed to determine the best attenuated virus to clonally amplify for future vaccine production. While this is the golden age of VZV research, much is still left to do.
Executive summary.
Varicella zoster virus latency in the postvaccine era
Following introduction of varicella vaccine, varicella-associated hospital complications have declined significantly.
Varicella vaccine contains attenuated virus from a clinical isolate and is a mixed population of mutated viruses. Research is now focussed on construction of a new vaccine based on a pure strain of attenuated virus or specific varicella zoster virus (VZV) proteins.
The varicella vaccine is associated with breakthrough disease, even in highly vaccinated populations, and the attenuated virus can establish latency and reactivate to cause, like the wild-type virus, zoster.
The zoster vaccine is also based on the attenuated virus and has greatly reduced the burden of illness from VZV reactivation.
There are many hurdles to achieving the goal of universal vaccination with the shingles vaccine, for example high vaccine cost, health insurance reimbursement policies, lack of education and vaccine storage requirements.
Even in this postvaccine era, VZV latency remains a concern, especially for elderly and immunocompromised people.
From primary infection to the ganglion
VZV DNA has been found in geniculate, vestibular, trigeminal, cervical, thoracic and sacral ganglia. Thus, VZV can establish latent infection in ganglia from all regions of the body.
HSV-1 is generally restricted to ganglia of the head and neck. HSV-1 DNA is present in geniculate, vestibular, olfactory and multiple cranial (especially trigeminal) ganglia, but only rarely in dorsal root ganglia.
Ganglion seeding with virus
Deletion of VZV open reading frames 4, 29 and 63 yields viruses that seed rat ganglion to significantly reduced levels compared with wild-type virus.
Multiple approaches confirmed that VZV, like HSV-1, resides in ganglionic neurons during latent infection.
The clinical differences between HSV-1 and VZV reactivation cannot be explained by fundamental differences in the cell-type, virus burden per ganglion or virus burden per cell in latently infected trigeminal ganglia.
Establishment & maintenance of the latent infection
During latency, both VZV and HSV-1 genomes are predominantly transcriptionally silent, and in an endless, circular, most probably single unit, episomal form.
The molecular events by which the VZV genome is silenced in the neuronal nucleus resulting in latency are still not fully understood. While ND-10-induced silencing of VZV gene expression is an attractive mechanism, significant work remains to be done.
VZV open reading frame 63p is the most likely candidate VZV gene expressed during latency to contain an antiapoptotic function.
VZV gene expression during latency is potential area that demands further research.
Clinical outcome of VZV reactivation
Zoster is typically a single event involving one–three dermatomes with residual pain and scarring lasting for many months, unlike HSV-1, which is associated with multiple, frequent and localized reactivations (cold sores).
VZV is the only herpesvirus directly associated with stroke.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported in part by Public Health Service grant AG032958 from the National Institutes of Health. Emily Eshleman was supported by The GEMS training grant R25HL103286–01 from the National Heart Lung and Blood Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Black FL, Woodall JP, Evans AS, Liebhaber H, Henle G. Prevalence of antibody against viruses in the Tiriyo, an isolated Amazon tribe. Am J Epidemiol. 1970;91:430–438. doi: 10.1093/oxfordjournals.aje.a121153. [DOI] [PubMed] [Google Scholar]
- 2.Ryan MA, Smith TC, Honner WK, Gray GC. Varicella susceptibility and vaccine use among young adults enlisting in the United States Navy. J Med Virol. 2003;70(Suppl 1):S15–S19. doi: 10.1002/jmv.10314. [DOI] [PubMed] [Google Scholar]
- 3.Wharton M. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am. 1996;10:571–581. doi: 10.1016/s0891-5520(05)70313-5. [DOI] [PubMed] [Google Scholar]
- 4••.Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA. 2005;294:797–802. doi: 10.1001/jama.294.7.797. Large-scale population analysis recommending a second immunization with attenuated varicella zoster virus (VZV) to protect against primary infection. [DOI] [PubMed] [Google Scholar]
- 5.Chaves SS, Gargiullo P, Zhang JX, et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356:1121–1129. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- 6.Donahue JG, Kieke BA, Yih WK, et al. Varicella vaccination and ischemic stroke in children: is there an association? Pediatrics. 2009;123:E228–E234. doi: 10.1542/peds.2008-2384. [DOI] [PubMed] [Google Scholar]
- 7••.Glanz JM, McClure DL, Magid DJ, et al. Parental refusal of varicella vaccination and the associated risk of varicella infection in children. Arch Pediatr Adolesc Med. 2010;164:66–70. doi: 10.1001/archpediatrics.2009.244. Original description of attenuated VZV for immunization use. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 9•.Breuer J, Schmid DS. Vaccine Oka variants and sequence variability in vaccine-related skin lesions. J Infect Dis. 2008;197(Suppl 2):S54–S57. doi: 10.1086/522140. Molecular description of the attenuated VZV vaccine and its relationship to wild-type virus. [DOI] [PubMed] [Google Scholar]
- 10.Schmid DS. Varicella-Zoster virus vaccine: molecular genetics. Curr Top Microbiol Immunol. 2010;342:323–340. doi: 10.1007/82_2010_14. [DOI] [PubMed] [Google Scholar]
- 11••.Jacquet A, Haumont M, Massaer M, et al. Immunogenicity of a recombinant varicella-zoster virus gE-IE63 fusion protein, a putative vaccine candidate against primary infection and zoster reactivation. Vaccine. 2002;20:1593–1602. doi: 10.1016/s0264-410x(01)00486-8. Original article describing the benefits of large-scale immunization with attenuated VZV to protect against zoster. [DOI] [PubMed] [Google Scholar]
- 12.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 13.Schmid DS, Jumaan AO. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev. 2010;23:202–217. doi: 10.1128/CMR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin MJ, DeBiasi RL, Bostik V, Schmid DS. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444–1447. doi: 10.1086/592452. [DOI] [PubMed] [Google Scholar]
- 15.Adams EN, Parnapy S, Bautista P. Herpes zoster and vaccination: a clinical review. Am J Health Syst Pharm. 2010;67:724–727. doi: 10.2146/ajhp090118. [DOI] [PubMed] [Google Scholar]
- 16.High KP. Overcoming barriers to adult immunization. J Am Osteopath Assoc. 2009;109:S25–S28. [PubMed] [Google Scholar]
- 17.Komara FA. Herpes zoster vaccination: benefits and barriers. J Am Osteopath Assoc. 2009;109:S22–S24. [PubMed] [Google Scholar]
- 18.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. [PubMed] [Google Scholar]
- 19.Harnisch JP. Zoster in the elderly: clinical, immunologic and therapeutic considerations. J Am Geriatr Soc. 1984;32:789–793. doi: 10.1111/j.1532-5415.1984.tb06298.x. [DOI] [PubMed] [Google Scholar]
- 20.Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:3361–3381. doi: 10.1128/cmr.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drwal-Klein LA, O’Donovan CA. Varicella in pediatric patients. Ann Pharmacother. 1993;27:938–949. doi: 10.1177/106002809302700723. [DOI] [PubMed] [Google Scholar]
- 22•.Preblud SR, D’Angelo LJ. Chickenpox in the United States, 1972–1977. J Infect Dis. 1979;140:257–260. doi: 10.1093/infdis/140.2.257. Concise analysis of methods to detect primary VZV infections and description of clinical changes resulting in varicella vaccination. [DOI] [PubMed] [Google Scholar]
- 23•.Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23–32. doi: 10.1086/653113. Molecular description of the initial stages of VZV infection in the skin. [DOI] [PubMed] [Google Scholar]
- 24.Abendroth A, Morrow G, Cunningham AL, Slobedman B. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J Virol. 2001;75:6183–6192. doi: 10.1128/JVI.75.13.6183-6192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow G, Slobedman B, Cunningham AL, Abendroth A. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J Virol. 2003;77:4950–4959. doi: 10.1128/JVI.77.8.4950-4959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Huch JH, Cunningham AL, Arvin AM, et al. Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection. J Virol. 2010;84:4060–4072. doi: 10.1128/JVI.01450-09. Only in vitro model of VZV infection of human skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor SL, Moffat JF. Replication of varicella-zoster virus in human skin organ culture. J Virol. 2005;79:11501–11506. doi: 10.1128/JVI.79.17.11501-11506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Desloges N, Schubert C, Wolff MH, Rahaus M. Varicella-zoster virus infection induces the secretion of interleukin-8. Med Microbiol Immunol. 2008;197:277–284. doi: 10.1007/s00430-007-0060-3. Meeting summary describing current VZV nomenclature. [DOI] [PubMed] [Google Scholar]
- 29.Breuer J, Grose C, Norberg P, Tipples G, Schmid DS. A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24–25 July 2008. J Gen Virol. 2010;91:821–828. doi: 10.1099/vir.0.017814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dlugosch D, Eis-Hubinger AM, Kleim JP, et al. Diagnosis of acute and latent varicella-zoster virus infections using the polymerase chain reaction. J Med Virol. 1991;35:136–141. doi: 10.1002/jmv.1890350212. [DOI] [PubMed] [Google Scholar]
- 31.Parker SP, Quinlivan M, Taha Y, Breuer J. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol. 2006;44:3911–3914. doi: 10.1128/JCM.00346-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt-Chanasit J, Olschlager S, Bialonski A, et al. Novel approach to differentiate subclades of varicella-zoster virus genotypes E1 and E2 in Germany. Virus Res. 2009;145:347–349. doi: 10.1016/j.virusres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 33••.Carpenter JE, Henderson EP, Grose C. Enumeration of an extremely high particle-to-PFU ratio for Varicella-zoster virus. J Virol. 2009;83:6917–6921. doi: 10.1128/JVI.00081-09. Currently accepted model for the lack of cell-free VZV produced in tissue culture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JJ, Zhu Z, Gershon AA, Gershon MD. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Dixit R, Tiwari V, Shukla D. Herpes simplex virus type 1 induces filopodia in differentiated P19 neural cells to facilitate viral spread. Neurosci Lett. 2008;440:113–118. doi: 10.1016/j.neulet.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh MJ, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391:176–181. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci USA. 2000;97:8146–8150. doi: 10.1073/pnas.97.14.8146. Historically classic work describing dermatomal distribution of zoster. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Head H, Campbell AW. The pathology of Herpes Zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Furuta Y, Takasu T, Fukuda S, et al. Detection of varicella-zoster virus DNA in human geniculate ganglia by polymerase chain reaction. J Infect Dis. 1992;166:1157–1159. doi: 10.1093/infdis/166.5.1157. [DOI] [PubMed] [Google Scholar]
- 40.Gilden DH, Vafai A, Shtram Y, et al. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983;306:478–480. doi: 10.1038/306478a0. [DOI] [PubMed] [Google Scholar]
- 41.Gilden DH, Gesser R, Smith J, et al. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes. 2001;23:145–147. doi: 10.1023/a:1011883919058. [DOI] [PubMed] [Google Scholar]
- 42.Hufner K, Arbusow V, Himmelein S, et al. The prevalence of human herpesvirus 6 in human sensory ganglia and its co-occurrence with alpha-herpesviruses. J Neurovirol. 2007;13:462–467. doi: 10.1080/13550280701447059. [DOI] [PubMed] [Google Scholar]
- 43.Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol. 1992;31:444–448. doi: 10.1002/ana.410310417. [DOI] [PubMed] [Google Scholar]
- 44.Richter ER, Dias JK, Gilbert JE, Atherton SS. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis. 2009;200:1901–1906. doi: 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arbusow V, Schulz P, Strupp M, et al. Distribution of herpes simplex virus type 1 in human geniculate and vestibular ganglia: implications for vestibular neuritis. Ann Neurol. 1999;46:416–419. doi: 10.1002/1531-8249(199909)46:3<416::aid-ana20>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 46.Liedtke W, Opalka B, Zimmermann CW, Lignitz E. Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J Neurol Sci. 1993;116:6–11. doi: 10.1016/0022-510x(93)90082-a. [DOI] [PubMed] [Google Scholar]
- 47.Mahalingam R, Wellish M, Wolf W, et al. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323:627–631. doi: 10.1056/NEJM199009063231002. [DOI] [PubMed] [Google Scholar]
- 48.Theil D, Horn AK, Derfuss T, et al. Prevalence and distribution of HSV-1, VZV, and HHV-6 in human cranial nerve nuclei III, IV, VI, VII, and XII. J Med Virol. 2004;74:102–106. doi: 10.1002/jmv.20152. [DOI] [PubMed] [Google Scholar]
- 49.Evans CA, Slavin HB, Berry GP. Studies on herpetic infection in mice: IV The effect of specific antibodies on the progression of the virus within the nervous system of young mice. J Exp Med. 1946;84:429–447. doi: 10.1084/jem.84.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Taha Y, Scott FT, Parker SP, Syndercombe Court D, Quinlivan ML, Breuer J. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43(10):1301–1303. doi: 10.1086/508539. Experimental result showing that latency can be established before the skin rash develops. [DOI] [PubMed] [Google Scholar]
- 51.Mahalingam R, Wellish M, Soike K, et al. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- 52.Rajcani J, Ciampor F. Experimental pathogenesis of non-lethal herpesvirus infection and the establishment of latency. Acta Virol. 1978;22:278–286. [PubMed] [Google Scholar]
- 53.Willey DE, Trousdale MD, Nesburn AB. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci. 1984;25:945–950. [PubMed] [Google Scholar]
- 54.Gordon Y, Gilden DH, Shtram Y, et al. A low thymidine kinase-producing mutant of herpes simplex virus type 1 causes latent trigeminal ganglia infections in mice. Arch Virol. 1983;76:39–49. doi: 10.1007/BF01315702. [DOI] [PubMed] [Google Scholar]
- 55.Caudill JW, Romanowski E, Raullo-Cruz T, Gordon YJ. Recovery of a latent HSV-1 thymidine kinase negative strain following iontophoresis and co-cultivation in the ocularly-infected rabbit model. Curr Eye Res. 1986;5:41–45. doi: 10.3109/02713688608995164. [DOI] [PubMed] [Google Scholar]
- 56.Gordon YJ, Gilden DM, Becker Y. HSV-1 thymidine kinase promotes virulence and latency in the mouse. Invest Ophthalmol Vis Sci. 1983;24:599–602. [PubMed] [Google Scholar]
- 57.Rubenstein R, Price RW. Replication of thymidine kinase deficient herpes simplex virus type 1 in neuronal cell culture: infection of the PC 12 cell. Arch Virol. 1983;78:49–64. doi: 10.1007/BF01310858. [DOI] [PubMed] [Google Scholar]
- 58.Katz JP, Bodin ET, Coen DM. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta. 2010;1799:246–256. doi: 10.1016/j.bbagrm.2009.12.001. In-depth description of histone modifications (epigenetic regulation) occurring on latent herpes simplex virus type 1 (HSV-1) DNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Honess RW, Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973;12:1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. Classic paper describing the kinetics of HSV-1 gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis. I Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tombacz D, Toth JS, Petrovszki P, Boldogkoi Z. Whole-genome analysis of pseudorabies virus gene expression by real-time quantitative RT-PCR assay. BMC Genomics. 2009;10:491. doi: 10.1186/1471-2164-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leib DA, Coen DM, Bogard CL, et al. Immediate–early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadzot-Delvaux C, Merville-Louis MP, Delree P, et al. An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J Neurosci Res. 1990;26:83–89. doi: 10.1002/jnr.490260110. [DOI] [PubMed] [Google Scholar]
- 65.Grinfeld E, Sadzot-Delvaux C, Kennedy PG. Varicella-zoster virus proteins encoded by open reading frames 14 and 67 are both dispensable for the establishment of latency in a rat model. Virology. 2004;323:85–90. doi: 10.1016/j.virol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 66••.Banfield BW, Bird GA. Construction and analysis of alphaherpesviruses expressing green fluorescent protein. Methods Mol Biol. 2009;515:227–238. doi: 10.1007/978-1-59745-559-6_15. First description of a method for introducing site-specific mutations into the VZV genome. [DOI] [PubMed] [Google Scholar]
- 67.Cohen JI, Seidel KE. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc Natl Acad Sci USA. 1993;90:7376–7380. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mallory S, Sommer M, Arvin AM. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Nagaike K, Mori Y, Gomi Y, et al. Cloning of the varicella-zoster virus genome as an infectious bacterial artificial chromosome in Escherichia coli. Vaccine. 2004;22:4069–4074. doi: 10.1016/j.vaccine.2004.03.062. Description of a bacterial artificial chromosome containing the entire VZV genome, designed to introduce site-specific mutations resulting in markerless virus. [DOI] [PubMed] [Google Scholar]
- 70.Tischer BK, Kaufer BB, Sommer M, et al. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J Virol. 2007;81:13200–13208. doi: 10.1128/JVI.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wussow F, Fickenscher H, Tischer BK. Red-mediated transposition and final release of the mini-F vector of a cloned infectious herpesvirus genome. PLoS ONE. 2009;4:E8178. doi: 10.1371/journal.pone.0008178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshii H, Somboonthum P, Takahashi M, Yamanishi K, Mori Y. Cloning of full length genome of varicella-zoster virus vaccine strain into a bacterial artificial chromosome and reconstitution of infectious virus. Vaccine. 2007;25:5006–5012. doi: 10.1016/j.vaccine.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z, Huang Y, Zhu H. A highly efficient protocol of generating and analyzing VZV ORF deletion mutants based on a newly developed luciferase VZV BAC system. J Virol Methods. 2008;148:197–204. doi: 10.1016/j.jviromet.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heineman TC, Cohen JI. Deletion of the varicella-zoster virus large subunit of ribonucleotide reductase impairs growth of virus in vitro. J Virol. 1994;68:3317–3323. doi: 10.1128/jvi.68.5.3317-3323.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen JI, Seidel KE. Absence of varicella-zoster virus (VZV) glycoprotein V does not alter growth of VZV in vitro or sensitivity to heparin. J Gen Virol. 1994;75(Pt 11):3087–3093. doi: 10.1099/0022-1317-75-11-3087. [DOI] [PubMed] [Google Scholar]
- 76.Cohen JI, Seidel K. Varicella-zoster virus (VZV) open reading frame 10 protein, the homolog of the essential herpes simplex virus protein VP16, is dispensable for VZV replication in vitro. J Virol. 1994;68:7850–7858. doi: 10.1128/jvi.68.12.7850-7858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato H, Pesnicak L, Cohen JI. Use of a rodent model to show that varicella-zoster virus ORF61 is dispensable for establishment of latency. J Med Virol. 2003;70(Suppl 1):S79–S81. doi: 10.1002/jmv.10326. [DOI] [PubMed] [Google Scholar]
- 78.Cohen JI, Seidel KE. Varicella-zoster virus open reading frame 1 encodes a membrane protein that is dispensable for growth of VZVin vitro. Virology. 1995;206:835–842. doi: 10.1006/viro.1995.1006. [DOI] [PubMed] [Google Scholar]
- 79.Cohen JI, Krogmann T, Bontems S, Sadzot-Delvaux C, Pesnicak L. Regions of the varicella-zoster virus open reading frame 63 latency-associated protein important for replication in vitro are also critical for efficient establishment of latency. J Virol. 2005;79:5069–5077. doi: 10.1128/JVI.79.8.5069-5077.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen JI. Rodent models of Varicella-Zoster virus neurotropism. Curr Top Microbiol Immunol. 2010;342:277–289. doi: 10.1007/82_2010_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen JI, Cox E, Pesnicak L, Srinivas S, Krogmann T. The varicella-zoster virus open reading frame 63 latency-associated protein is critical for establishment of latency. J Virol. 2004;78:11833–11840. doi: 10.1128/JVI.78.21.11833-11840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ambagala AP, Krogmann T, Qin J, Pesnicak L, Cohen JI. A varicella-zoster virus mutant impaired for latency in rodents, but not impaired for replication in cell culture. Virology. 2010;399(2):194–200. doi: 10.1016/j.virol.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoover SE, Cohrs RJ, Rangel ZG, et al. Downregulation of varicella-zoster virus (VZV) immediate-early ORF62 transcription by VZV ORF63 correlates with virus replication in vitro and with latency. J Virol. 2006;80:3459–3468. doi: 10.1128/JVI.80.7.3459-3468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baiker A, Bagowski C, Ito H, et al. The immediate-early 63 protein of Varicella-Zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J Virol. 2004;78:1181–1194. doi: 10.1128/JVI.78.3.1181-1194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sadzot-Delvaux C, Debrus S, Nikkels A, et al. Varicella-zoster virus latency in the adult rat is a useful model for human latent infection. Neurology. 1995;12(Suppl 8):S18–S20. doi: 10.1212/wnl.45.12_suppl_8.s18. [DOI] [PubMed] [Google Scholar]
- 86.Tenser RB, Hyman RW. Latent herpesvirus infections of neurons in guinea pigs and humans. Yale J Biol Med. 1987;60:159–167. [PMC free article] [PubMed] [Google Scholar]
- 87.Rock DL, Nesburn AB, Ghiasi H, et al. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987;61:3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Deatly AM, Spivack JG, Lavi E, Fraser NW. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci USA. 1987;84:3204–3208. doi: 10.1073/pnas.84.10.3204. First detection of latent VZV in neurons of human ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stevens JG, Haarr L, Porter DD, Cook ML, Wagner EK. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988;158:117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- 90.Croen KD, Ostrove JM, Dragovic LJ, Straus SE. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci USA. 1988;85:9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kennedy PG, Steiner I. A molecular and cellular model to explain the differences in reactivation from latency by herpes simplex and varicella-zoster viruses. Neuropathol Appl Neurobiol. 1994;20:368–374. doi: 10.1111/j.1365-2990.1994.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 92••.Cohrs RJ, Wischer J, Essman C, Gilden DH. Characterization of varicella-zoster virus gene 21 and 29 proteins in infected cells. J Virol. 2002;76:7228–7238. doi: 10.1128/JVI.76.14.7228-7238.2002. Conclusive in situ results demonstrating latent VZV is found in neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennedy PG, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci USA. 1998;95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LaGuardia JJ, Cohrs RJ, Gilden DH. Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J Virol. 1999;73:8571–8577. doi: 10.1128/jvi.73.10.8571-8577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95••.Levin MJ, Cai GY, Manchak MD, Pizer LI. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77:6979–6987. doi: 10.1128/JVI.77.12.6979-6987.2003. Conclusive PCR results showing that latent VZV is in neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang K, Lau TY, Morales M, Mont EK, Straus SE. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol. 2005;79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98•.Sawtell NM, Poon DK, Tansky CS, Thompson RL. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998;72:5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. Largest study to date of latent VZV in human samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inoue H, Motani-Saitoh H, Sakurada K, et al. Detection of varicella-zoster virus DNA in 414 human trigeminal ganglia from cadavers by the polymerase chain reaction: a comparison of the detection rate of varicella-zoster virus and herpes simplex virus type 1. J Med Virol. 2010;82:345–349. doi: 10.1002/jmv.21687. [DOI] [PubMed] [Google Scholar]
- 100.Friedmann A, Becker Y. Circular and circular-linear DNA molecules of herpes simplex virus. J Gen Virol. 1977;37:205–208. doi: 10.1099/0022-1317-37-1-205. [DOI] [PubMed] [Google Scholar]
- 101.Friedmann A, Shlomai J, Becker Y. Electron microscopy of herpes simplex virus DNA molecules isolated from infected cells by centrifugation in CsCl density gradients. J Gen Virol. 1977;34:507–522. doi: 10.1099/0022-1317-34-3-507. [DOI] [PubMed] [Google Scholar]
- 102.Jacob RJ, Roizman B. Anatomy of herpes simplex virus DNA VIII. Properties of the replicating DNA. J Virol. 1977;23:394–411. doi: 10.1128/jvi.23.2.394-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Strang BL, Stow ND. Circularization of the herpes simplex virus type 1 genome upon lytic infection. J Virol. 2005;79:12487–12494. doi: 10.1128/JVI.79.19.12487-12494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jackson SA, DeLuca NA. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc Natl Acad Sci USA. 2003;100:7871–7876. doi: 10.1073/pnas.1230643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mellerick DM, Fraser NW. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987;158:265–275. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- 106•.Burkham J, Coen DM, Weller SK. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J Virol. 1998;72:10100–10107. doi: 10.1128/jvi.72.12.10100-10107.1998. Initial description of ND10s in HSV-1 infected cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107••.Maul GG, Ishov AM, Everett RD. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology. 1996;217:67–75. doi: 10.1006/viro.1996.0094. Complete review describing the interaction of promyelocytic leukemia protein (ND10) with HSV-1 DNA. [DOI] [PubMed] [Google Scholar]
- 108.Saffert RT, Kalejta RF. Promyelocytic leukemia-nuclear body proteins: herpesvirus enemies, accomplices, or both? Future Virol. 2008;3:265–277. doi: 10.2217/17460794.3.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Everett RD, Rechter S, Papior P, et al. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J Virol. 2006;80:7995–8005. doi: 10.1128/JVI.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Everett RD, Murray J, Orr A, Preston CM. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J Virol. 2007;81:10991–11004. doi: 10.1128/JVI.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McMahon R, Walsh D. Efficient quiescent infection of normal human diploid fibroblasts with wild-type herpes simplex virus type 1. J Virol. 2008;82:10218–10230. doi: 10.1128/JVI.00859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Everett RD, Boutell C, McNair C, Grant L, Orr A. Comparison of the biological and biochemical activities of several members of the alphaherpesvirus ICP0 family of proteins. J Virol. 2010;84:3476–3487. doi: 10.1128/JVI.02544-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kyratsous CA, Silverstein SJ. Components of nuclear domain 10 bodies regulate varicella-zoster virus replication. J Virol. 2009;83:4262–4274. doi: 10.1128/JVI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clarke P, Beer T, Cohrs R, Gilden DH. Configuration of latent varicella-zoster virus DNA. J Virol. 1995;69:8151–8154. doi: 10.1128/jvi.69.12.8151-8154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang L, Voytek CC, Margolis TP. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J Virol. 2000;74:209–217. doi: 10.1128/jvi.74.1.209-217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ozaki N, Sugiura Y, Yamamoto M, et al. Apoptosis induced in the spinal cord and dorsal root ganglion by infection of herpes simplex virus type 2 in the mouse. Neurosci Lett. 1997;228:99–102. doi: 10.1016/s0304-3940(97)00364-9. [DOI] [PubMed] [Google Scholar]
- 117.Doerig C, Pizer LI, Wilcox CL. An antigen encoded by the latency-associated transcript in neuronal cell cultures latently infected with herpes simplex virus type 1. J Virol. 1991;65:2724–2727. doi: 10.1128/jvi.65.5.2724-2727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Henderson G, Jaber T, Carpenter D, Wechsler SL, Jones C. Identification of herpes simplex virus type 1 proteins encoded within the first 1.5 kb of the latency-associated transcript. J Neurovirol. 2009;15:439–448. doi: 10.3109/13550280903296353. [DOI] [PubMed] [Google Scholar]
- 119.Drolet BS, Perng GC, Cohen J, et al. The region of the herpes simplex virus type 1 LAT gene involved in spontaneous reactivation does not encode a functional protein. Virology. 1998;242:221–232. doi: 10.1006/viro.1997.9020. [DOI] [PubMed] [Google Scholar]
- 120.Perng GC, Jones C, Ciacci-Zanella J, et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 121.Farrell MJ, Dobson AT, Feldman LT. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA. 1991;88:790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zabolotny JM, Krummenacher C, Fraser NW. The herpes simplex virus type 1 2.0-kilobase latency-associated transcript is a stable intron which branches at a guanosine. J Virol. 1997;71:4199–4208. doi: 10.1128/jvi.71.6.4199-4208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang W, Mukerjee R, Gartner JJ, et al. Characterization of a spliced exon product of herpes simplex type-1 latency-associated transcript in productively infected cells. Virology. 2006;356:106–114. doi: 10.1016/j.virol.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 124•.Mitchell WJ, Lirette RP, Fraser NW. Mapping of low abundance latency-associated RNA in the trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Gen Virol. 1990;71(Pt 1):125–132. doi: 10.1099/0022-1317-71-1-125. Clinical evidence for simultaneous VZV and HSV-1 reactivation. [DOI] [PubMed] [Google Scholar]
- 125.Giehl KA, Muller-Sander E, Rottenkolber M, Degitz K, Volkenandt M, Berking C. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. J Eur Acad Dermatol Venereol. 2008;22(6):722–728. doi: 10.1111/j.1468-3083.2008.02587.x. [DOI] [PubMed] [Google Scholar]
- 126•.Cohrs RJ, Laguardia JJ, Gilden D. Distribution of latent herpes simplex virus type-1 and varicella zoster virus DNA in human trigeminal Ganglia. Virus Genes. 2005;31:223–227. doi: 10.1007/s11262-005-1799-5. Evidence that HSV-1 and VZV can be latent within the same human neuronal cell. [DOI] [PubMed] [Google Scholar]
- 127.Theil D, Paripovic I, Derfuss T, et al. Dually infected (HSV-1/VZV) single neurons in human trigeminal ganglia. Ann Neurol. 2003;54:678–682. doi: 10.1002/ana.10746. [DOI] [PubMed] [Google Scholar]
- 128.Kennedy PG, Grinfeld E, Bell JE. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J Virol. 2000;74:11893–11898. doi: 10.1128/jvi.74.24.11893-11898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meier JL, Holman RP, Croen KD, Smialek JE, Straus SE. Varicella-zoster virus transcription in human trigeminal ganglia. Virology. 1993;193:193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- 130.Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grinfeld E, Kennedy PG. Translation of varicella-zoster virus genes during human ganglionic latency. Virus Genes. 2004;29:317–319. doi: 10.1007/s11262-004-7434-z. [DOI] [PubMed] [Google Scholar]
- 132.Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133••.Mahalingam R, Wellish M, Cohrs R, et al. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. First indication that immune surveillance during latency differs between HSV-1 and VZV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Theil D, Derfuss T, Paripovic I, et al. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mahalingam R, Kennedy PG, Gilden DH. The problems of latent varicella zoster virus in human ganglia: precise cell location and viral content. J Neurovirol. 1999;5:445–448. doi: 10.3109/13550289909045372. [DOI] [PubMed] [Google Scholar]
- 136•.Cohrs RJ, Srock K, Barbour MB, et al. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J Virol. 1994;68:7900–7908. doi: 10.1128/jvi.68.12.7900-7908.1994. Cloning and sequence verification of VZV transcripts present in latently infected human trigeminal ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137•.Cohrs RJ, Barbour M, Gilden DH. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J Virol. 1996;70:2789–2796. doi: 10.1128/jvi.70.5.2789-2796.1996. Identification of VZV ORF66 transcripts and protein in latently infected human ganglia and possible mechanistic implications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138•.Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J Virol. 2003;77:6660–6665. doi: 10.1128/JVI.77.12.6660-6665.2003. Quantitative analysis of latent VZV trancripts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hood C, Cunningham AL, Slobedman B, et al. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J Virol. 2006;80:1025–1031. doi: 10.1128/JVI.80.2.1025-1031.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zerboni L, Sobel RA, Ramachandran V, et al. Expression of varicella-zoster virus immediate-early regulatory protein IE63 in neurons of latently infected human sensory ganglia. J Virol. 2010;84:3421–3430. doi: 10.1128/JVI.02416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maue AC, Haynes L. CD4+ T cells and immunosenescence – a mini-review. Gerontology. 2009;55:491–495. doi: 10.1159/000214842. [DOI] [PubMed] [Google Scholar]
- 143.Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol. 2010;142(Suppl 1):S39–S44. doi: 10.1016/j.jcpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 144.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009;21:440–445. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 145.Koch S, Larbi A, Ozcelik D, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann NY Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 146.Hayward AR, Herberger M. Lymphocyte responses to varicella zoster virus in the elderly. J Clin Immunol. 1987;7:174–178. doi: 10.1007/BF00916011. [DOI] [PubMed] [Google Scholar]
- 147.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–1156. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- 149•.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by Varicella Zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–253. doi: 10.1007/82_2009_3. Initial demonstration that VZV reactivation is associated with space flight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mehta SK, Cohrs RJ, Forghani B, et al. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 151.Furuta Y, Ohtani F, Fukuda S, Inuyama Y, Nagashima K. Reactivation of varicella-zoster virus in delayed facial palsy after dental treatment and oro-facial surgery. J Med Virol. 2000;62:42–45. [PubMed] [Google Scholar]
- 152.Thompson RL, Sawtell NM. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J Virol. 2006;80:10919–10930. doi: 10.1128/JVI.01253-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Paulus C, Nitzsche A, Nevels M. Chromatinisation of herpesvirus genomes. Rev Med Virol. 2010;20:34–50. doi: 10.1002/rmv.632. [DOI] [PubMed] [Google Scholar]
- 154•.Placek BJ, Berger SL. Chromatin dynamics during herpes simplex virus-1 lytic infection. Biochim Biophys Acta. 2010;1799:223–227. doi: 10.1016/j.bbagrm.2010.01.012. Possible mechanistic description of HSV-1 reactivation. [DOI] [PubMed] [Google Scholar]
- 155.Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5:E1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.apRhys CM, Ciufo DM, O’Neill EA, Kelly TJ, Hayward GS. Overlapping octamer and TAATGARAT motifs in the VF65-response elements in herpes simplex virus immediate-early promoters represent independent binding sites for cellular nuclear factor III. J Virol. 1989;63:2798–2812. doi: 10.1128/jvi.63.6.2798-2812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Triezenberg SJ, LaMarco KL, McKnight SL. Evidence of DNA: protein interactions that mediate HSV-1 immediate early gene activation by VP16. Genes Dev. 1988;2:730–742. doi: 10.1101/gad.2.6.730. [DOI] [PubMed] [Google Scholar]
- 158.Hufner K, Derfuss T, Herberger S, et al. Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J Neuropathol Exp Neurol. 2006;65:1022–1030. doi: 10.1097/01.jnen.0000235852.92963.bf. [DOI] [PubMed] [Google Scholar]
- 159•.Knickelbein JE, Khanna KM, Yee MB, et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. Description of immune surveillance of latent HSV-1 and VZV in human ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jin L, Perng GC, Carpenter D, et al. Reactivation phenotype in rabbits of a herpes simplex virus type 1 mutant containing an unrelated antiapoptosis gene in place of latency-associated transcript. J Neurovirol. 2007;13:78–84. doi: 10.1080/13550280601164333. [DOI] [PubMed] [Google Scholar]
- 162.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lungu O, Annunziato PW, Gershon A, et al. Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc Natl Acad Sci USA. 1995;92:10980–10984. doi: 10.1073/pnas.92.24.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- 166.Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gowrishankar K, Steain M, Cunningham AL, et al. Characterization of the host immune response in human ganglia after herpes zoster. J Virol. 2010;84(17):8861–8870. doi: 10.1128/JVI.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Steain M, Gowrishankar K, Rodriguez M, Slobedman B, Abendroth A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella zoster virus infection. J Virol. 2011;85(1):626–631. doi: 10.1128/JVI.01816-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crow KD, Ellis JP. Shingles: a belt of roses from hell. Br Med J. 1979;1(6155):5. [PMC free article] [PubMed] [Google Scholar]
- 170.Gilden DH, Mahalingam R, Cohrs RJ, Kleinschmidt-Demasters BK, Forghani B. The protean manifestations of varicella-zoster virus vasculopathy. J Neurovirol. 2002;8(Suppl 2):75–79. doi: 10.1080/13550280290167902. [DOI] [PubMed] [Google Scholar]
- 171.Miravet E, Danchaivijitr N, Basu H, Saunders DE, Ganesan V. Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev Med Child Neurol. 2007;49:417–422. doi: 10.1111/j.1469-8749.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 172.Nagel MA, Mahalingam R, Cohrs RJ, Gilden D. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets. 2010;10:105–111. doi: 10.2174/187152610790963537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Doyle PW, Gibson G, Dolman CL. Herpes zoster ophthalmicus with contralateral hemiplegia: identification of cause. Ann Neurol. 1983;14:84–85. doi: 10.1002/ana.410140115. [DOI] [PubMed] [Google Scholar]
- 175.Fujiwara Y, Yanagihara N, Kurata T. Middle ear mucosa in Ramsay Hunt syndrome. Ann Otol Rhinol Laryngol. 1990;99(5 Pt 1):359–362. doi: 10.1177/000348949009900508. [DOI] [PubMed] [Google Scholar]
- 176.Umbach JL, Nagel MA, Cohrs RJ, Gilden DH, Cullen BR. Analysis of human alphaherpesvirus microRNA expression in latently infected human trigeminal ganglia. J Virol. 2009;83(20):10677–10683. doi: 10.1128/JVI.01185-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci USA. 2005;102(18):6490–6495. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zerboni L, Hinchliffe S, Sommer MH, et al. Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology. 2005;332(1):337–346. doi: 10.1016/j.virol.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 179.Zerboni L, Reichelt M, Jones CD, Zehnder JL, Ito H, Arvin AM. Aberrant infection and persistence of varicella-zoster virus in human dorsal root ganglia in vivo in the absence of glycoprotein I. Proc Natl Acad Sci USA. 2007;104(35):14086–14091. doi: 10.1073/pnas.0706023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82(8):3971–3983. doi: 10.1128/JVI.02592-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ecker JR, Hyman RW. Varicella zoster virus DNA exists as two isomers. Proc Natl Acad Sci USA. 1982;79(1):156–160. doi: 10.1073/pnas.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vrabec JT, Alford RL. Quantitative analysis of herpes simplex virus in cranial nerve ganglia. J Neurovirol. 2004;10:216–222. doi: 10.1080/13550280490463569. [DOI] [PubMed] [Google Scholar]
- 183.Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hill JM, Ball MJ, Neumann DM, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]