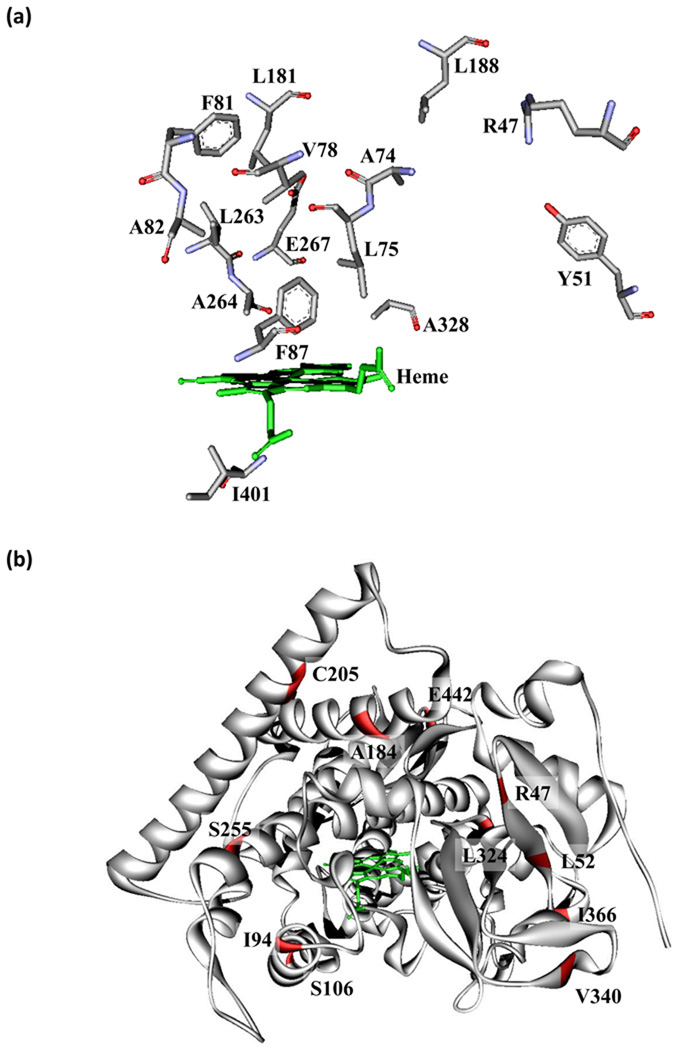
Figure 2.
Crystal structure showing the residues of P450 BM3 altering substrate recognition and thermostability. (a) Stick representations are shown of 15 active site residues distal to the heme which can undergo mutation and alter substrate scope and selectivity. (b) Residues that improve thermostability are colored red on the crystal structure (PDB: 1BU7).