Abstract
Primary cilia are microtubule-based organelles that serve as hubs for the transduction of various developmental signaling pathways including Hedgehog, Wnt, FGF and PDGF. Ciliary dysfunction contributes to a range of disorders, collectively known as the ciliopathies. Recently, interest has grown in these syndromes, particularly among craniofacial biologists, as many known and putative ciliopathies have severe craniofacial defects. Herein we discuss the current understanding of ciliary biology and craniofacial development in an attempt to gain insight into the molecular etiology for craniofacial ciliopathies, and uncover a characteristic ciliopathic craniofacial gestalt.
Keywords: craniofacial, ciliopathy, hypertelorism, midline defects, Shh, Wnt
Introduction
Facial appearance has long been used to help reveal the etiology of severe developmental disorders. For trained physicians who see a multitude of patients, being able to identify a characteristic facial gestalt is often the key to early diagnosis. With a structure as diverse as the craniofacial complex, identifying common phenotypes among syndromes is a difficult task. When found however, a characteristic facial symptom can be a powerful tool in determining the molecular etiology of a syndrome, and more importantly can serve to characterize syndromes into a class of disorders that may have some variability, but can be linked by phenotypic hallmarks.
The primary cilia (also known as sensory or immotile cilia) are evolutionarily conserved organelles that have recently gained notoriety because of their association with a number of syndromes (Badano et al., 2006). Primary cilia were first observed in the renal epithelium and thyroid gland (Zimmermann, 1898), but it is now understood that they are present on almost all vertebrate cell types. The ubiquitous nature of this organelle allows for insight into its functionality in a number of cellular processes. Notably, cilia have emerged as important regulators of developmental signaling pathways (Eggenschwiler and Anderson, 2007), suggesting that a deeper understanding of these structures can inform our comprehension of developmental processes.
Primary cilia have become important factors to consider when determining the etiology of craniofacial disorders for two main reasons. First, a number of disorders identified as ciliopathies have craniofacial abnormalities including Oro-facial-digital syndrome, Joubert syndrome, Bardet-Biedl syndrome, Meckel-Gruber syndrome, Ellis-van Creveld syndrome and the recently identified ciliopathy, Sensenbrenner Syndrome (Table 1). Second, primary cilia are essential for proper signal transduction of molecular pathways that are necessary for normal craniofacial growth including the Hedgehog (Hh), Fibroblast Growth Factor (FGF), Wnt and Platelet-derived growth factor (PDGF) pathways. Based on these two facts, it is reasonable to examine primary cilia in craniofacial disorders with no known cause. Herein, we review both ciliary biology and craniofacial development, with the aims of better understanding the etiology of the phenotypes associated with both known and postulated craniofacial ciliopathies, and defining a characteristic ciliopathic facial phenotype.
Table 1.
Established ciliopathies with craniofacial phenotpes
| Syndrome | Gene | Phenotype | References |
| Oro-facial-digital syndrome |
OFD1 | facial asymmetry, hypertelorism, micrognathia, broadened nasal ridge, hypoplasia of the malar bones and nasal alar cartilages, frontal bossing, pseudocleft, cleft palate, hamartomas of the tongue, bifid tongue, hyperplastic oral frenula |
(Gurrieri et al., 2007) (Ferrante et al., 2001) |
| Joubert syndrome |
MKS3 NPHP6 RPGRIP1L AH1 ARL13B |
prominent forehead, high rounded eyebrows, epicanthal folds, ptosis, upturned nose with evident nostrils, open mouth and tongue protrusion and rhythmic tongue motions |
(Maria et al., 1999) (Baala et al., 2007) (Delous et al., 2007) (Sayer et al., 2006) (Cantagrel et al., 2008) |
| Bardet-Biedl syndrome |
BBS1-12 MKS1 MKS3 |
mid-face abnormalities, nasal bridge hypoplasia, reduced length/bulbosity of the nasal tip, mid-facial shortening and flattening, mild retrognathia |
(Tobin et al., 2008) reviewed in (Zaghloul and Katsanis, 2010) |
| Meckel-Gruber syndrome |
MKS1 MKS3 |
encephalocele, neural tube closure defects, cleft lip and palate |
reviewed in (Zaghloul and Katsanis, 2010) (Dawe et al., 2007) (Leitch et al., 2008) |
| Ellis-van Creveld syndrome |
EVC1 EVC2 |
hypertrophy labiogingival frenulum, upper lip abnormalities, premature eruption of teeth and the presence of teeth at birth |
(McKusick et al., 1964) (Ruiz-Perez et al., 2000) (Ruiz-Perez et al., 2003) |
| Cranioectodermal Dysplasia/ Sensenbrenner syndrome |
IFT122 WDR35 |
sagittal craniosyntosis, epicanthal folds, hypodontia or microdontia, everted lip, multiple oral frenula, high arched palate, skeletal and ectodermal anomalies |
(Levin et al., 1977) (Sensenbrenner et al., 1975) (Walczak-Sztulpa et al., 2010) (Gilissen et al., 2010) |
Primary cilia: Structure and function
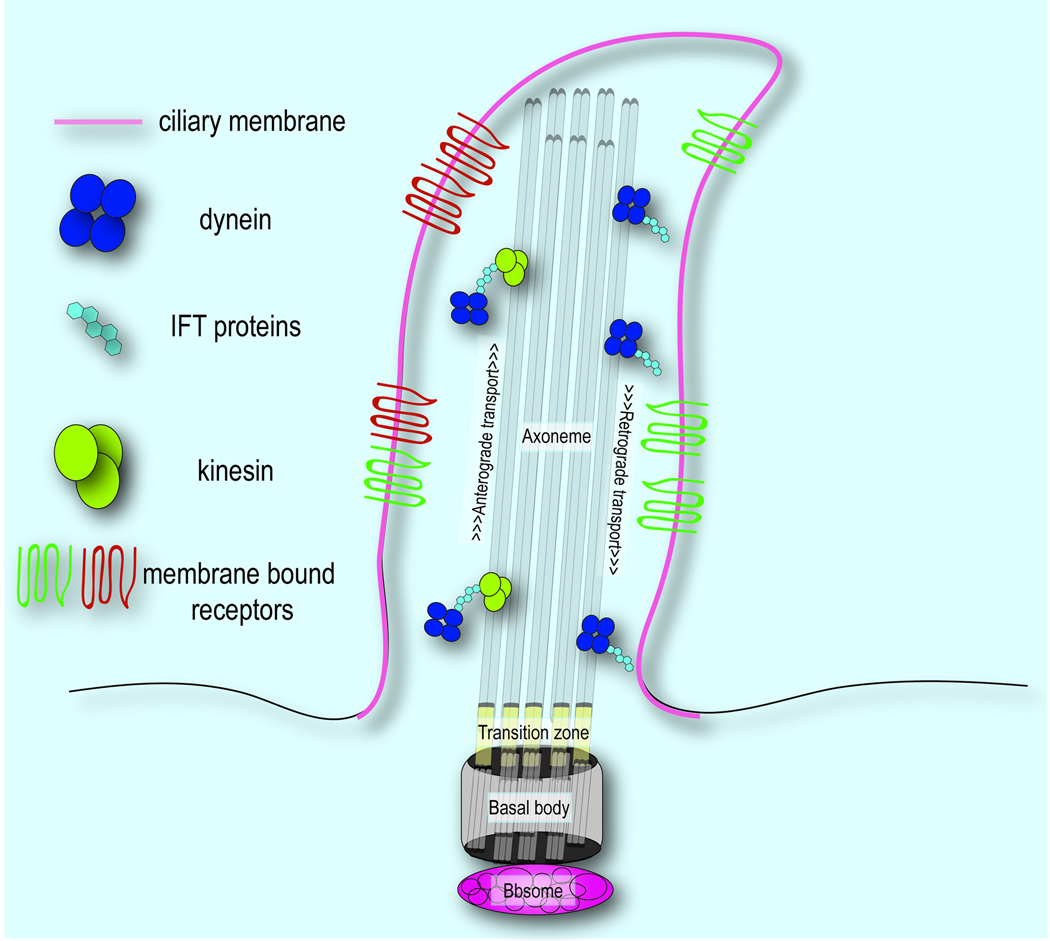
Primary cilia extend from the apical surface of cells and are characterized by three main components the basal body, a microtubule-based structure that anchors the cilia to the cell body and has several known functions; the axoneme, a microtubule extension comprised of rings of 9 microtubule doublets; and the ciliary membrane, a receptor laden extension of the cellular membrane that ensheaths the axoneme. We will briefly discuss the function of each structure and discuss the known proteins that localize to them.
The basal body
With respect to cilia, the basal body serves as not only the nucleation point from which ciliogenesis occurs, but also as the mediator of cargo transport from the cytoplasm to the ciliary membrane (reviewed in (Marshall, 2008)). A plethora of proteins localize to the basal body and are necessary for ciliogenesis and cilia function. The most well understood class of these proteins is the BBS proteins, associated with Bardet-Biedl Syndrome (BBS), which form a complex known as the BBSome. The BBSome associates with Rab8 via Rabin8 to regulate transport to the ciliary membrane, perhaps by the formation of coated vesicles (Nachury et al., 2007; Jin et al., 2010). As such, though loss of BBS protein function does not impair ciliogenesis, processes requiring interaction between ciliary and cytoplasmic proteins are impaired.
Other basal body proteins regulate interactions with ciliary proteins and their loss results in ciliary dysfunction. For example, animals devoid of RPGRIP1L (FTM), a basal body protein mutated across many ciliopathies (Delous et al., 2007; Khanna et al., 2009), produce fewer cilia with inefficient transduction of cilia-specific Sonic hedgehog (Shh) signaling (Vierkotten et al., 2007). RPGRIP1L also interacts with another basal body protein, NPHP4, which contributes to nephronophthisis (NPHP) and Senor-Loken Syndrome (SLS), suggesting a joint role for these proteins in regulation of basal body function (Arts et al., 2007). Likewise, CEP290/NPHP6, mutated in NPHP, BBS, SLS, Meckel-Gruber Syndrome (MKS), and Joubert Syndrome (JBTS) (reviewed in (Zaghloul and Katsanis, 2010)) interacts with other basal body proteins and regulates transport of Rab8 to the cilium (Kim et al., 2008). Thus, the basal body represents an important structure serving as a mediator of ciliary protein interactions that are central to ciliary function.
The axoneme/IFT
Proteins transported to the cilium are then shuttled along the axoneme by a specialized system of transport known as intraflagellar transport (IFT). Mediated by complexes consisting of motor proteins and IFT proteins, ciliary cargo is transported from the basal body to the tip of the axoneme (anterograde IFT) as well as in the reverse direction (retrograde IFT). The kinesin-2 motor protein complexes with IFT complex B proteins to direct anterograde transport, while dynein complexes with IFT A proteins in retrograde transport (reviewed in (Gerdes et al., 2009)). This process is critical for ciliogenesis and the proper localization of proteins to the cilium. Thus, defects in IFT proteins often produce severe consequences, such as complete loss of cilia, and consequent phenotypic abnormalities.
The ciliary membrane
In addition to the basal body and axoneme, the ciliary membrane plays a crucial role in ciliary function, particularly in the reception and transduction of extracellular signaling cues. A growing list of membrane-bound receptors localize to the cilium and are necessary for binding of ligands and subsequent communication to downstream effectors. The first to be identified, and best understood, is the localization of the Shh receptors, Patched (Ptch1) and Smoothened (Smo). Binding of Shh to Ptch results in its removal from the ciliary membrane, allowing Smo to localize there (Corbit et al., 2005; Rohatgi et al., 2007). Similarly, other transmembrane receptors, including PDGFRα, G-protein coupled receptors, and extracellular matrix (ECM) receptors, have been observed on the cilium suggesting the importance of this specialized region of the cellular membrane for reception of extracellular signals (Schneider et al., 2005; McGlashan et al., 2006; Berbari et al., 2008). These findings provide a potential explanation for the loss or disruption of ciliary function leading to widespread ramifications for tissue specification and homeostasis. In the following sections we will review craniofacial development keeping the relationship to primary cilia in the forefront.
Development of the craniofacial complex
The craniofacial complex is the region comprised of the face and skull. The face develops as a result of the growth and seamless fusion of three-paired swellings (or prominences), and one medial singular swelling. The skull, comprised of neurocranium (skull vault) and the basicranium (skull base), arises through the fusion of various bones. Whereas each of the facial prominences is composed of three or more distinct tissues of various origins, the skull is derived from mesenchyme (neural crest or mesodermal in origin) that requires developmental signals from surrounding tissues. We briefly introduce the craniofacial prominences, their tissue components and explain the origin of dysmorphologies commonly present in craniofacial ciliopathies.
The frontonasal prominence
The frontonasal prominence (FNP), also known as the medial nasal prominence, is the singular-medial swelling comprised of neuroectoderm, cranial neural crest (NCC) and facial (surface) ectoderm. The FNP is unique in the fact that it is the only singular facial prominence. The FNP gives rise to the forehead, bridge and tip of the nose, the philtrum, the medial portion of the upper lip, and the primary palate. The FNP first fuses to the maxillary prominence, then to the lateral nasal prominence to create the seamless fusion between the midface and more lateral aspect of the face (Mooney and Siegel, 2002). Aberrant growth of the FNP results in many easily recognizable defects. Collapse (reduced growth) or expansion (increased growth) of the FNP creates conditions clinically referred to as hypotelorism or hyperterlorism, respectively. More localized growth disruptions in the FNP cause a failure of fusion between the frontonasal and the adjacent facial prominences, the lateral nasal and/or maxillary prominences. In scenarios such as these, cleft lip or palate is the phenotypic consequence. Midfacial defects and clefts are among some of the most common dysmorphologies associated with craniofacial ciliopathies (see Table 1 & Table 2).
Table 2.
Possible Craniofacial Ciliopathies
| Syndrome | Gene | Phenotype |
|---|---|---|
| Acromelic frontonasal dysostosis | Gli3 | anterior cranium bifidum, severe hypertelorism, median cleft lip and palate, nasal bifurcation, brachycephaly, large fontanelle |
| Acrofacial dysostosis | abnormal dental position, flat cheek bones, high forehead, high nasal bridge, narrow-high arched palate, long upper lip groove, low set ears, microcephaly, micrognathia, facial cleft |
|
| Branchio-oculo-facial syndrome | TFAP2A | dolichocephaly, malformed pinnae, flat-thick nasal tip, up-slanted eyes, pseudocleft upper lip, microdontia, broad nasal bridge |
| C syndrome | CD96 | Trigonocephaly,craniosynostosis, narrow-pointed forehead, flat-broad nasal bridge, short nose, vertical folds over inner corners of nose, deeply furrowed palate, anomalous and posteriorly angulated ears |
| Carpenter syndrome | Rab23 | sagittal craniosynostosis, micrognathia,low-set malformed ears, flat nasal bridge, wide upturned nose, downslanting palpebral fissures |
| Cerebrofaciothoracic dysplasia | cleft lip palate, ocular hypertelorism, macrocephaly, brachycephaly, low hair line,narrow forehead, synophrys, epicanthal folds, low set ears, flat face, short neck |
|
| Cerebrooculonasal syndrome | absent eyes, cleft lip, high-arched palate, proboscis-like nose, large forehead, flat brow, epicanthic folds, underdeveloped cheek bones,large upper lip groove, single upper incisor, abnormal external ears,postaxial polydactyly, ocular hypertelorism |
|
| Craniofrontonasal syndrome | EFNB1 | ocular hypertelorism, coronal craniosynostosis, craniofacial asymmetry, frontal bossing, downslanting palpebral fissures, broad bifid nose, low posterior hairline with an anterior widow’s peak, cleft lip/palate |
| Dysgnathia complex | microstomia, microglossia, aglossia, micrognathia, agnathia,ocular hypertelorism, low set ears | |
| Ectrodactyly–ectodermal dysplasia–cleft syndrome (EEC1) |
maxillary hypoplasia, microcephaly, malformed ear, furrowed tongue | |
| Frontonasal dysplasia | ocular hypertelorism, nasal clefting, anterior cranium bifidum occultum | |
| Fryns syndrome | abnormal ear shape, cleft lip/palate, macrostomia, microretrognathia, broad nasal bridge, forward tilting nostrils | |
| Greig cephalopolysyndactyly | Gli3 | high forehead, frontal bossing, macrocephaly, ocular hypertelorism, broad nasal root, down-turned corners of mouth |
| Hypothalamic hamartomas | hydrocephalus, encephalocele, macrocephaly | |
| Kabuki syndrome | long palpebral fissures, arched eyebrows, prominent eyelashes, prominent and/or misshapen ears, depressed nasal tip |
|
| Lenz-Majewski hyperostotic dwarfism |
large cranium, broad prominent forehead, late closure of large fontanels, ocular hypertelorism, ptosis | |
| Oculoauriculofrontonasal syndrome |
microtia, preauricular tags, hemifacial microsomia, lateral face clefting, upper palpebral colobomata | |
| Oculodentodigital dysplasia | Connexin- 43 |
ocular hypertelorism, small thin nose, anteverted nostrils, hypodontia, wide lower jaw |
| Otopalatodigital syndrome 2 | Filamin A | delayed closure of anterior fontanel, prominent forehead, wide sutures, low-set ears, malformed ears, ocular hypertelorism, flat nose bridge, microstomia, micrognathia (mandibular), cleft palate, excess mineralization of skull base, under-mineralization of cranial vault, large gonial angle, broad face, midface hypoplasia |
| Pitt-Hopkins syndrome | TCF-4 | thick lips, macrostomia, microcephaly, wide-high arched palate, broad nose, micropthalmia, beaked nose, ocular hypertelorism, Cupid’s bow upper lip |
| Robinow syndrome | Ror-2 | macrocephaly, large anterior fontane, frontal bossing, ocular hypertelorism, ptosis, down-slanting palpebral fissures, small upturned nose, long philtrum,triangular down turned mouth, micrognathia (mandibular), hyperplastic alveolar ridges |
| Schinzel-Giedion midface- retraction |
high protruding forehead, short nose, low nasal bridge, forward tilting nostrils, shallow orbits, drooping eyelid, ocular hypertelorism, midface hypoplasia, steep short skull base |
|
| Ven Den Ende-Gupta syndrome | narrow eye slits, narrow-beaked nose, micrognathia (maxillary), cleft palate, everted lower lip,flat cheek bones, triangular face |
The lateral nasal prominences
The lateral nasal prominences (LNPs) are paired prominences that sit lateral to the FNP and medial to the maxillary prominences. The LNPs give rise to the outer wall of the nostrils, the alae of the nose. The development of the olfactory placode/nasal pit initiates the separation of the FNP and LNP. This separation removes the LNP from being in direct contact with forebrain, and thus it is not under the same molecular influences as the FNP. Although rare in nature, clefts that involve the alae often result from aberrant mesenchymal portioning between the lateral and frontonasal prominences (Bluestone, 2003) This disruption in mesenchymal migration upsets the growth the prominences and prevents fusion between the lateral nasal prominences and either the frontonasal or maxillary processes.
The maxillary prominences
The maxillary prominences give rise to the upper jaw and the sides of the face, the sides of the upper-lip, and the secondary palate. The maxillary prominences arise from the first branchial arch and are composed of a mesodermally derived core surrounded by cranial neural crest cells (NCCs). This mesenchymal core is encased by surface ectoderm externally and pharyngeal endoderm internally. Perturbations in the growth and fusion of the maxillary prominences frequently result in a cleft lip or cleft palate. Furthermore, maxillary growth defects can also produce maxillary hypoplasia (maxillary micrognathia). Cleft lip/palate and maxillary hypoplasia are extremely common in established and likely ciliopathies (Table 1 & Table 2) and also in animal models with disrupted cilia (Table 3).
Table 3.
Animal models for craniofacial ciliopathies
| Model system |
Mutant/Morphant Name |
Gene | Craniofacial phenotype | References |
|---|---|---|---|---|
| Mouse | Slb | Ift172 | Holoprosencephaly, exencephaly |
(Gorivodsky et al., 2009) |
| Kif3a–Wnt1-Cre |
Kif3a in cranial NCCs |
anterior cranium occultum, bifid nasal septum, aglossia, severe hypertelorism, dental abnormalities |
(Brugmann et al., 2010) | |
| Henin | Arl13b | exencephaly | (Caspary et al., 2007) | |
| Orpk | Ift88 | dental abnormalities, micrognathia, increased gonial angle |
(Ohazama et al., 2009) | |
| Ofd1−/− | Ofd1 | severe cleft palate and shortened skull and facial regions |
(Ferrante et al., 2006) | |
| Evc−/− | Evc | chondrodysplasia, hypodontia |
(Ruiz-Perez et al., 2007) | |
| Hippi−/− | Ift57 | exencephaly, abnormal cranial flexure, hypotelorism and abnormal maxillary processes |
(Houde et al., 2006) | |
| Dnchc2W2502R | Dnchc2 | hypotelorism | (May et al., 2005) | |
| Dnchc2GT | Dnchc2 | exencephaly | (Huangfu and Anderson, 2005) | |
| Rpgrip1 | Rpgrip1/Ftm | exencephaly, microphthalmia, rounded skull, cleft upper lip, micrognathia |
(Delous et al., 2007) | |
| Ift122−/− | Ift122 | exencephaly, anencephaly, altered eye development, defects of the ventral portion of the head, altered development of branchial arches |
(Cortellino et al., 2009) | |
| Ift52hypo | Ift52 | single median nostril, fused maxillary prominences, exencephaly |
(Liu et al., 2005) | |
| Mks | Mks1 | Occipital meningoencephalocoele, hypomineralization and/or splitting of the frontal, parietal and supraoccipital bones, hydrocephaly, cleft palate |
(Weatherbee et al., 2009) | |
| Kif7−/− | kif7 | microphthalmia, exencephaly |
(Endoh-Yamagami et al., 2009) | |
| Intu | Inturned | exencephaly, microphthalmia, reduced telencephalon, enlarged diencephalon |
(Zeng et al., 2010) | |
| Fuz | Fuz | exencephaly, anophthalmia, encephalocoele, mandibular defects, micrognathia |
(Gray et al., 2009) | |
| Aln | Ift139 | delayed eye and forebrain development and neural tube defects |
(Tran et al., 2008) | |
| Bbs6 | Bbs6 | mid-facial shortening due to premaxillary and maxillary hypoplasia |
(Tobin et al., 2008) | |
| Chicken | Talpid2 | unknown | rounded head, shortened or absent upper beak, hypotelorism, cleft lip, trapezoidal shaped mouth |
(Brugmann et al., 2010) |
| Talpid3 | Talpid3 | Ocular hypotelorism, frontonasal hypoplasia, fused maxillary prominences, micrognathia |
(Yin et al., 2009) | |
| Zebrafish | Ofd1 morphant | ofd1 | abnormal blunting of the jaw, disorganization of Meckel’s cartilage cells |
(Ferrante et al., 2009) |
| Ift80 morphant | ift80 | abnormal anterior neurocranial development, midline fusion |
(Beales et al., 2007) | |
| Bbs morphants |
bbs4, bbs6, bbs8 |
mandibular dysplasia, severe craniofacial reduction, anteroneurocranium hypoplasia |
(Tobin et al., 2008) | |
| Ift mutants |
ift57, ift88, ift172 |
mild dysmorphology of craniofacial cartilages |
(Lunt et al., 2009) | |
| Ift122 morphant | ift122 | reduced ocular development, distended cranium consistent with hydrocephalus, otolith defects |
(Walczak-Sztulpa et al., 2010) |
The mandibular prominences
The mandibular prominence forms the lower jaw, lower lip and the anterior portion of the tongue. Similar to the maxillary prominence, the mandibular prominence is derived from the first branchial arch and is composed of mesoderm, NCCs, surface ectoderm and pharyngeal endoderm. Although mandibular micrognathia is common, clefts of the lower jaw occur with far less frequency than those of the upper jaw and can range in severity from a pseudocleft to a complete cleft of the lip involving the tongue, chin and supporting structures of the neck (Ishii et al., 2002). Defects involving tongue (glossal) development and mandibular micrognathia are associated with established and likely ciliopathies (Table 1 & Table 2) and also in animal models with disrupted cilia (Table 3).
The skull
The neurocranium is the area of the skull that surrounds the brain. Bones of the skull are derived from mesenchyme of both mesodermal and NCC origin. Although the skull is derived from mesenchyme, proper development of the skull requires molecular signals emanating from the underlying neuroectoderm and overlying surface ectoderm (reviewed in (Lenton et al., 2005b; Rice, 2008)). Disrupted growth of the skull can cause severe and far-reaching craniofacial phenotypes. Insufficient growth and ossification of the skull results in a condition referred to as cranium bifidum in which the brain is exposed through a hole in the skull (encephalocele). Exuberant growth and ossification of the skull results in a condition called craniosynostosis: the premature fusion of one or more cranial sutures. This premature fusion of any of the bones of the cranial vault disrupts the overall growth of the skull by inducing compensatory growth in the parallel direction of the original defect (Cohen, 2005). As such, craniosynostosis results in a number of morphological and functional abnormalities, including dysmorphic cranial vault and facial asymmetry (Marchac and Renier, 1989; Posnick, 2000; Lenton et al., 2005a). A number of known and predicted ciliopathies exhibit either cranium bifidum or craniosynostosis (see Table 1 & Table 2). Both conditions are also present in ciliopathic animal models (Table 3).
The tissues of the craniofacial complex
As mentioned, each of the facial prominences is made up of various tissue components. Extended primary cilia have been identified in vivo on many of these components. We briefly review the tissue components of the craniofacial complex and speak to the observed effect loss of cilia has on tissue development.
Neuroectoderm
The association between the brain and the face has been of interest to craniofacial biologist for decades (DeMyer, 1964). The neuroectoderm participates in craniofacial development by contributing to the FNP. Not only does the forebrain act as a structural support for facial development, it also acts as a signaling center, as many of the signals that pattern the FNP originate in the forebrain (Cohen and Sulik, 1992; Muenke, 1994). The role of primary cilia is probably best understood in the neuroectoderm (Huangfu et al., 2003; Huangfu and Anderson, 2005; Caspary et al., 2007) because of the established role of Shh in pattering the neural tube and generating various cell fates (Marti et al., 1995). Utilizing the neuroectoderm to gain insight into how cilia function was essential in jumpstarting the field and it highlighted the fact that the relationship between cilia and various signaling pathways is not a binary one.
Analysis of the neural tube in various ciliary mutants reveals a range of defects. Anterograde IFT mutants including Ift88−/− or Ift172−/− embryos have reduced Shh signaling and thus a dorsalized neural tube (Huangfu et al., 2003). This phenotype is less severe than a complete loss of Shh because the loss of cilia prevents the processing of the Gli3 repressor, thus the net affect is similar to losing both transduction and repression of the Hedgehog pathway. As a result, some cell types that require low levels of Hedgehog signaling are specified. Dynein motor mutants display a similar phenotype as those seen in anterograde IFT mutants (reviewed in (Goetz and Anderson, 2010).
In contrast, retrograde IFT mutants exhibit phenotypes opposite to those seen in their anterograde counterparts. Ift139−/− mutants show excessive Shh signaling and a ventralized neural tube (Tran et al., 2008). Furthermore, Ift122−/− mutants show a significant, yet moderate activation of the Shh activity, with a modest expansion motor neurons and other cell types that require intermediate levels of Hedgehog signaling (Cortellino et al., 2009); reviewed in (Goetz and Anderson, 2010)). Taken together these findings support a hypothesis that the classification of the IFT protein affected has a distinct role in Shh processing and neural patterning.
Reviewing these data from a craniofacial biologist perspective sheds light on the craniofacial phenotypic variation frequently associated with ciliopathies. The forebrain serves as a signaling center that secretes morphogens that influence facial outgrowth. Expression of Shh in the forebrain controls midfacial width, and growth and polarity of the upper jaw (Hu et al., 2003; Marcucio et al., 2005b; Hu and Marcucio, 2009). Reduced Shh signaling in the forebrain produces a hypoteloric phenotype with a narrow and truncated face (Cordero et al., 2004; Marcucio et al., 2005b). Conversely, increased Shh signaling in the forebrain produces a hyperteloric phenotype with a wide upper jaw and face (Hu and Marcucio, 2009). Furthermore, the relationship between levels of Shh in the forebrain and the width of the face appears to be dose dependent in nature (Young et al., 2010). Midline defects are among the most common dysmorphologies associated with ciliopathies, both in human and animal model systems (Table 1,Table 2 & Table 3). The association between cilia, Shh, neuroectoderm and the face elucidates why this phenotype is so prevalent in ciliopathies, and makes a case for midline abnormalities to serve as a characteristic craniofacial ciliopathic phenotype.
Cranial neural crest
NCCs are an ectomesenchymal population of cells that give rise to the majority of the facial skeleton and skull. They are of ectodermal origin, as they are born at the interface between the neuroectoderm and surface ectoderm (Selleck and Bronner-Fraser, 1995). NCCs migrate along established pathways to invade all of the facial prominences. Once NCCs have reached their final destination they receive local cues and proliferate to support the growth of each prominence. Primary cilia have been identified on NCC in both mice and fish (Tobin et al., 2008; Brugmann et al., 2010).
Disruption of primary cilia affects NCC behavior. Loss of cilia as a result of a Bbs mutation in zebrafish results in an inability of NCCs to migrate ventrally into the facial prominences. Interestingly this ciliary requirement for NCC migration is not seen in mutations of other ciliary genes, in other species (Brugmann et al., 2010). Conditional loss of cilia due to loss of Kif3a in NCCs produces a severe facial phenotype that phenocopies human frontonasal dysplasia by presenting with a bifid nasal septum, anterior cranium occultum, aglossia, dental anomalies, brain defects and severe hypertelorism. This phenotype, one of severe hypertelorism, is consistent with a gain of Hedgehog function (Vortkamp et al., 1991; Hu and Helms, 1999). This result was initially surprising because in the neural tube loss of Kif3a results in phenotypes consistent with a loss of Hedgehog function, including a loss of ventral cell types specified by high levels of Shh ((Huangfu and Anderson, 2005) reviewed by (Goetz and Anderson, 2010)). In contrast, the hyperteloric phenotype seen in the conditional Kif3a knockout in NCCs, Hedgehog pathway activity is expanded within the NCCs and this correlates with the hyper-proliferation of midline NCCs (Brugmann et al., 2010). The molecular mechanism behind the onset of this phenotype will be discussed in a later section discussing the Hedgehog pathway. Taken together, these finding suggest that the loss of cilia has a unique and significant effect on NCC development. The exact effects seen are dependent upon which ciliary gene is disrupted, and may be species-specific.
Surface ectoderm
The tissue encasing all of the facial prominences is the surface (or facial) ectoderm. This ectoderm is also a source of organizing signals that direct the underlying mesenchyme to proliferate and differentiate into the skeletal tissues of the face. Shh expression is activated in the facial ectoderm when neural crest cells migrate into the facial prominences. This facial ectoderm, referred to as the ‘facial ectodermal zone’ (FEZ), acts as a signaling center that controls outgrowth of facial prominences (Marcucio et al., 2005a). Furthermore, proper molecular patterning in the facial ectoderm is essential for the growth and development of ectodermally derived structures like teeth, hair and whiskers (reviewed in (Chuong and Noveen, 1999))
Primary cilia have been detected in facial ectoderm (Brugmann et al., 2010), however the exact role of cilia in this tissue remains nebulous. Mice harboring a conditional deletion of Polaris specifically in the facial mesenchyme die at birth, exhibiting severe craniofacial abnormalities and supernumerary teeth. In contrast, mice with a conditional deletion of Polaris specifically in the ectoderm survive and show no evidence of supernumerary teeth, although they do have polydactyly (Ohazama et al., 2009). It is interesting to note that although the loss of cilia in the underlying mesenchyme affects gene expression in the overlying oral ectoderm, loss of cilia in the ectoderm itself does not cause the same mispatterning. These findings highlight the possible role primary cilia may play in regulating signaling between tissues.
Recently, various ectodermal dysplasias have been classified as, and purported to be, ciliopathies (Konstantinidou et al., 2009; Walczak-Sztulpa et al., 2010). In syndromes like these, common ectodermal phenotypes include dental abnormalities, sparse hair, skin laxity and abnormal nails (Konstantinidou et al., 2009; Walczak-Sztulpa et al., 2010). Further understanding of the relationship between ectoderm and cilia will undoubtedly shed light on the etiology of this class of disorders.
Pharyngeal endoderm, prechordal plate and paraxial mesoderm
The pharyngeal endoderm serves as the inner lining of the branchial arches and gives rise to tonsils, thymus, parathyroid and auditory tubes. The pharyngeal endoderm acts as an important signaling center during development of the arches via localized expression of mitogens and morphogens including BMPs, FGFs, and Hhs (Graham and Smith, 2001). The prechordal plate mesoderm gives rise to the voluntary muscles of the head, whereas the paraxial mesoderm gives rise to the more posterior bones of the skull. To date, the role of primary cilia during pharyngeal endoderm and/or prechordal plate and paraxial mesoderm development has not been extensively studied. Given the compiled data, it is possible that these tissues may interpret loss of various ciliary proteins in their own unique way.
Cilia and signaling in craniofacial development
With the recognition of the role of primary cilia as central hubs of signal transduction for various developmental pathways essential for craniofacial patterning, the implications for mechanistic links between cilia and craniofacial development are significant. Craniofacial development relies heavily on Hedgehog, Wnt, and other pathways for proper morphogenesis. To dissect the role of cilia during craniofacial development, it will be necessary to understand the role each pathway plays in normal morphogenesis and superimpose the role cilia play in the regulation of these pathways. In doing so we hope to elucidate the etiology of some craniofacial ciliopathies and understand the involvement of ciliary proteins associated with craniofacial disorders.
Hedgehog signaling
The Hedgehog family of proteins has numerous roles in a developmental context. During craniofacial development the most notable Hedgehog is Shh. Shh acts as both a mitogen and a morphogen during craniofacial development and is integral to NCC survival, proliferation and patterning (Chiang et al., 1996; Ahlgren and Bronner-Fraser, 1999; Hu and Helms, 1999; Cordero et al., 2004; Jeong et al., 2004; Young et al., 2010). The expression of Shh in the ventral neuroectoderm, facial ectoderm and foregut endoderm is essential for such processes. Perturbations in Shh most frequently affect patterning of the facial midline. Reductions in Shh activity result in a spectrum of diseases characterized by a midline hypoplasia including hypotelorism and various forms of holoprosencephaly including cyclopia (Belloni et al., 1996; Chiang et al., 1996; Roessler et al., 1996). At the other end of the spectrum, amplification of Shh signaling produces midline expansions including hypertelorism and midline duplications (diprosopus) (Ming et al., 1998; Hu and Helms, 1999) (Balk and Biesecker, 2008).
The first indication of the cilium as a conduit for the Hedgehog pathway came with the identification of the requirement of anterograde and retrograde IFT for Shh signaling in the ift172, ift88, kif3a and dync2h1 mouse mutants (Huangfu et al., 2003; May et al., 2005). Further investigation revealed that the Ptch and Smo receptors localize to the cilium (Corbit et al., 2005) and Gli3, which functions as an effector of the pathway, requires some IFT proteins for proper proteolytically processing into it’s functional repressor. This conclusion was reached via in vitro experiments using kif3a null murine fibroblasts. To determine if Gli3 was being processed correctly, levels of full-length versus repressor form were analyzed in kif3a+/+ and kif3a−/− cells. Levels of full-length Gli3 were similar in both kif3a+/+ and kif3a−/− cells, however kif3a−/− cells had significantly lower levels of processed Gli3 repressor (Gli3R) (Humke et al., 2010). A similar situation occurs in vivo, in the limb. In control limb buds, full-length Gli3 is efficiently cleaved into the truncated repressor form, this processing is impaired in the kif3a−/− limb mesenchyme (Kolpakova-Hart et al., 2007). Although there is no evidence that Kif3a directly acts on Gli3, the net molecular consequence of losing kif3a in a both murine fibroblasts and the developing limb is similar to a gain of function phenotype because the action of the Gli3R was lost. It must be noted that the loss of other IFT proteins in other tissues produces a loss of hedgehog phenotype (Goetz and Anderson, 2010). Thus, the most accurate statement regarding the loss of cilia and Hedgehog signaling is that a loss of cilia alters Hedgehog signal transduction. The exact alteration of the pathway is dependent upon which ciliary protein is perturbed, and in which tissue the perturbation occurs.
Given the key role of Shh in craniofacial development and the recent implication of IFT genes in craniofacial disease, there is strong evidence for a link between the two. Ift122 null mice, for example, exhibit defects of the ventral portion of the head and altered development of branchial arches in addition to impairment of Shh signaling (Cortellino et al., 2009). Kif3a null mice display reduced branchial arches, among other defects (Marszalek et al., 1999). Furthermore, mice lacking kif3a specifically in NCCs have a widened frontonasal prominence and clefting of the secondary palate, reminiscent of human frontonasal dysplasia (Brugmann et al., 2010). As previously mentioned, these defects are accompanied by an increase of NCC proliferation as a result of increased Hedgehog responsiveness and expression. This may be due to ineffectual cleavage of Gli3 into its repressor form, as was observed in mice lacking kif3a specifically in Wnt1-expressing NCCs (Kolpakova-Hart et al., 2007). Thus, the tissue affected and the form of Gli predominant in that tissue, can determine how a loss of cilia alters Hedgehog signaling.
Other IFT mutants also exhibit defects reminiscent of perturbations in the Shh pathway. Ift88 mutants produce ectopic teeth as a result of expanded Shh signaling (Ohazama et al., 2009). Interestingly, zebrafish ift57, ift88 and ift172 mutants and morphants exhibit very modest craniofacial defects and no defects in Hedgehog signaling. These findings suggest that the IFT requirement for Hedgehog processing may be a mammalian specific feature (Lunt et al., 2009). Thus, in addition to accounting for which tissue is affected and which form of Gli is predominant, species must also be taken into account when evaluating the relationship between IFT proteins and Hedgehog signaling.
Like IFT proteins, disruption of basal body proteins perturbs Shh signaling, giving rise to craniofacial defects. Rpgrip1l, Mks1, and Ofd1 knockout mice lack cilia and exhibit midfacial defects, consistent with perturbed Shh signaling (Ferrante et al., 2006; Delous et al., 2007; Vierkotten et al., 2007; Weatherbee et al., 2009; Bimonte et al., 2010) (Table 3). Other members of the Hedgehog pathway are also affected by the loss of ciliary proteins. Evc has been implicated as a positive mediator of Indian hedgehog (Ihh) regulated bone growth (Ruiz-Perez et al., 2007). Evc is localized to the base of the cilium and is preferentially expressed in the developing bones and the orofacial region. Evc−/− mice develop EVC-like craniofacial symptoms including dental abnormalities. Although Ihh is expressed normally in these mutants, expression of the Ihh downstream genes Ptch1 and Gli1 is significantly reduced. Studies using Evc−/− cells demonstrate that the defect lies downstream of Smo and that Evc is an intracellular component of the Hedgehog signal transduction pathway that is required for normal transcriptional activation of Ihh target genes (Ruiz-Perez et al., 2007). Taken together, these studies implicate the importance of basal body proteins in regulating Hedgehog signaling, particularly in the context of craniofacial development. This raises the possibility of the involvement of other basal body proteins known to be associated with craniofacial phenotypes, such as the BBS proteins, with Hedgehog regulation.
Wnt signaling
Wnt signaling regulates a cadre of diverse developmental processes, including cell proliferation, determination, differentiation, and survival (reviewed in (Cadigan and Nusse, 1997)). Wnt signaling also plays an important role in the generation and migration of NCCs (Schmidt and Patel, 2005; Basch and Bronner-Fraser, 2006), and the development and regionalization of the face in various species (Brugmann et al., 2007). Several members of the Wnt family, as well as downstream transcription factors of the pathway, are expressed in the developing facial prominences (Oosterwegel et al., 1993; Wang and Shackleford, 1996; Geetha-Loganathan et al., 2009) and the subsequent pathway activity is specifically localized to facial epithelia and the underlying mesenchyme in the lateral nasal, maxillary and mandibular prominence, as demonstrated by various Wnt reporter mice (BAT-gal and TOP-gal) (DasGupta and Fuchs, 1999, Maretto, 2003 #6579).
The hypothesized role for Wnt activity in the facial prominences varies within different tissues. In the NCC mesenchyme Wnts promote proliferation, thus promoting the growth of the facial prominences (Brugmann et al., 2007). In the facial epithelium, expression of multiple Wnt is essential for the fusion of facial prominences (Lan et al., 2006; Geetha-Loganathan et al., 2009; Song et al., 2009). Susceptibility to clefting is linked to disruptions in various Wnt genes (Juriloff et al., 1996; Juriloff et al., 2001; Juriloff et al., 2005) and perturbations of the pathway produce mild to severe facial clefting in various animal models (Brugmann et al., 2007; Song et al., 2009), as well as in humans (Chiquet et al., 2008).
In addition to clefting, both micrognathia and hypertelorism are common phenotypes in Wnt signaling defects. Based on the suggested role for Wnt in promoting proliferation in the mesenchyme of both the maxillary and mandibular prominences, it is not difficult to consider that a disruption of the pathway could lead to reduced growth of the upper and lower jaw. The reduced growth of the maxillary prominence eliminates the growth constraints on the FNP and allows for midline expansion (Brugmann et al., 2007), thus the onset of hypertelorism.
The link between cilia and regulation of Wnt signaling is somewhat less clear than that of Hedgehog, in part due to the lack of obvious localization of Wnt receptors to the ciliary membrane. However, mounting evidence suggests the involvement of ciliary proteins in regulating downstream components of the pathway and the switch between canonical and non-canonical signaling. This role is in large part due to the interaction with Disheveled (Dvl), a protein that drives the canonical β-catenin-dependent pathway when localized to the nucleus and the non-canonical planar cell polarity (PCP) pathway when membrane bound (Veeman et al., 2003). Disruption of ciliogenesis by suppression of kif3a produces a heightened Wnt-driven transcriptional response, as a result of constitutive Dvl phosphorylation by casein kinase I (Corbit et al., 2008). Similarly, down-regulation of BBS proteins in mammalian cells or zebrafish results in slight upregulation of the canonical Wnt response with a concomitant suppression of the non-canonical PCP pathway, possibly through BBS regulation of β-catenin degradation at the level of the proteasome (Gerdes et al., 2007). Another basal body protein, Inversin (NPHP2) inhibits canonical Wnt signaling and promotes the PCP pathway by targeting cytoplasmic Dvl for degradation (Simons et al., 2005). These findings are corroborated by the presence of convergent extension defects, a hallmark of defective PCP signaling, in BBS and OFD1 morphant zebrafish (Ross et al., 2005; Ferrante et al., 2009). Recently, conflicting evidence has emerged in mouse and zebrafish IFT mutants where no Wnt defects were observed (Huang and Schier, 2009; Ocbina et al., 2009). These conflicting data may suggest protein, tissue or species-specific differences in the role of cilia in regulating Wnt signaling.
With respect to craniofacial features, recent studies link BBS proteins to Wnt-mediated regulation of NCCs in zebrafish. Perturbation of non-canonical Wnt signaling in Bbs morphants disrupted NCC migration into the facial prominences. The disrupted ventral migration could be partially rescued by co-injection of a truncated form of Dvl, which induces the non-canonical Wnt pathway (Tobin et al., 2008). Taken together these data provide a potential mechanism by which ciliary disruption could disrupt Wnt signaling and cause craniofacial defects.
FGF signaling
Members of the FGF family are key factors in craniofacial development and disease. FGF ligands have been implicated in the growth and development of facial prominences as well as development of the facial skeleton (Trumpp et al., 1999; Macatee et al., 2003; Creuzet et al., 2004; Szabo-Rogers et al., 2008). In particular, Fgf8 is expressed in the frontonasal and mandibular prominences early in facial development followed by tightly localized expression around the maxillary and mandibular prominences and the nasal pits (Bachler and Neubuser, 2001). Furthermore, FGF receptors have been implicated in suture development and gain of function mutations in these receptors are frequently reported causes for craniosynostosis (reviewed in (Lenton et al., 2005a)), a phenotype present in various predicted ciliopathies (see Table 2).
Recent studies suggest that FGF signaling is essential for regulating cilia length and function (Neugebauer et al., 2009). Disruption of FGF signaling through the Fgf receptor 1 (Fgfr1) causes reduced expression of ift88 and two transcription factors important in controlling ciliary gene expression, foxj1 and rfx2. In a zebrafish model system, fgfr1 mutations result in phenotypes similar to those seen in ift88 mutants, including curved body axis, kidney cysts and shortened cilia. The current hypothesis suggests ligands that signal through Ffgr1 including Fgf8 and Fgf2/4 activate the receptor cell-autonomously to maintain a transcriptional network that allows normal expression of IFT proteins required for normal length cilia (Neugebauer et al., 2009). Findings such as these support the possibility that some craniofacial malformations that have characteristics common to disruptions in the FGF pathway may actually be ciliopathic in nature.
PDGF signaling
Platelet-derived growth factor (PDGF) and its receptors (PDGFR) are important regulators during embryonic development. They have specific roles in promoting tissue–tissue interactions to control cell migration, proliferation and survival (reviewed in (Hoch and Soriano, 2003)). In regards to craniofacial development, PDGFR-alpha expression is specifically associated with NCC and placode development (Soriano, 1997; McCabe and Bronner-Fraser, 2008). Disruptions in the PDGF pathway have severe repercussions on craniofacial development. Deletion of Pdgfra in the murine NCCs leads to defects in palatal closure and fusion, nasal septation, and the development of several facial bone and cartilage structures (Morrison-Graham et al., 1992; Soriano, 1997; Tallquist and Soriano, 2003). Deletion of the ligands Pdgfa and Pdgfc also produce severe craniofacial phenotypes. Both ligands are highly expressed the epithelial lining of the branchial arches and branchial pouches (Orr-Urtreger and Lonai, 1992; Aase et al., 2002), indicating a role as migration cues or post-migratory signals for NCCs. Pdgfc null neonates have a complete cleft of the secondary palate accompanied by the failure of the palatal bones to extend across the roof of the oronasal cavity. Pdgfc/Pdgfa double knockout embryos develop a cleft face with subepidermal blistering and cranial bone defects (Ding et al., 2004).
PDGF signaling through the PDGFRαα receptor requires intact cilia. The receptor localizes to the cilium and fails to be upregulated and activated in Ift88 mutants lacking normal cilia (Schneider et al., 2005). This defect can prevent cells from migrating properly (Schneider et al., 2010). Though this has only been specifically shown for fibroblasts in a wound healing response and remains to be seen in other cell types, defects in PDGF-dependent NCC migration suggest a possible mechanism by which ciliary defects could perturb the PDGF pathway resulting in craniofacial defects.
Linking cilia and craniofacial development: Is there a common phenotype for craniofacial ciliopathies?
Outside of OFD and EVC, craniofacial features have not historically been considered among the primary diagnostic criteria for ciliopathies. However, increasing recognition of the role of cilia in regulating signaling transduction and cell migration during craniofacial development has turned attention to the face. With the understanding of ciliopathies growing, a phenotypic classification scheme is beginning to emerge. There is growing support for the proposition that ciliopathies are associated with a higher degree of phenotypic similarity than non-ciliopathies (Baker and Beales, 2009). While diagnosis based on phenotypic presentation is impossible, a recent review defines and identifies nine core characteristics (retinitis pigmentosa, renal cystic disease, polydactyly, mental retardation, situs inversus, agenesis of the corpus callosum, Dandy-Walker malformation, posterior encephalocele and hepatic disease) that are more likely present in a ciliopathy rather than a disorder not caused by ciliary dysfunction (Baker and Beales, 2009). In addition, less globally penetrant phenotypes, such as obesity, insulin resistance/diabetes, cardiovascular defects and skeletal anomalies, have also been reported in patients with ciliopathies (Baker and Beales, 2009).
Beales and colleagues hypothesize that the greater number of these core features within any individual, the higher the likelihood of a ciliopathy. Using these core characteristics as criteria of a ciliopathy they profiled two disease databases (London Dysmorphology Database and OMIM) for syndromes that presented with some combination of the nine core characteristics. They identified 102 conditions with a unique combination of the core characteristics that are either known, or what they considered possible ciliopathies. When evaluating the disorders presented by Beales and colleagues as known, likely and potential ciliopathies, we find that approximately 30% of the disorders put forth are defined primarily by their craniofacial phenotype (Table 1 and Table 2). Of those disorders, approximately 70% present with midfacial defects, specifically hypertelorism- the widening of the midface. Can midfacial dysmorphology be used as a hallmark of a craniofacial ciliopathy? Perhaps that link is a bit simplistic and premature at present, but given the relationship between cilia, important craniofacial signaling pathways and midfacial development, the appearance of midfacial defects in unclassified craniofacial syndromes should warrant the examination of ciliary genes in patients.
The prevalence of midfacial defects in known and potential ciliopathies is not very surprising given the established role of cilia in Shh and Wnt signal transduction. Indeed, deciphering ciliary regulation of these pathways will likely play a large part in our understanding of ciliary involvement in craniofacial patterning. It will be critical to understand, for example, if perturbed ciliary function gives rise primarily to Shh defects, Wnt defects or both. Evidence in both mouse and zebrafish models point to the disruption of both pathways in the developing face of ciliary mutants and morphants (Tobin et al., 2008; Brugmann et al., 2010). Given the differential observations of Shh and Wnt defects in different studies of ciliary mutants (Huang et al., 2009; Lunt et al., 2009), it remains to be seen if cilia are necessary for one pathway and producing secondary deficits in the other, or if the cilium truly acts a coordinating center for morphogenetic signaling response. Initial studies linking PDGF and cilia point to the latter possibility, suggesting a complexity of interplay between ciliary proteins and the coordinated regulation of multiple, key pathways.
An additional challenge will be to decipher the differential contributions of ciliary proteins. Available animal models used to study craniofacial features in ciliary mutants vary between IFT particles, IFT motor proteins, and basal body components (Table 3). In addition to differential effects on ciliogenesis (i.e. shortened vs. absent), suppression of different proteins can produce different phenotypes. For example, kif3a knockout mice display severe craniofacial anomalies, however no such defects have been reported in bbs knockout mice (Mykytyn et al., 2004; Nishimura et al., 2004; Fath et al., 2005). In patients, the majority of disorders exhibiting primary craniofacial defects are caused by mutations in basal body proteins, such as OFD1, EVC or BBS. Sensenbrenner syndrome, a ciliopathy caused by a mutation in IFT122 (Walczak-Sztulpa et al., 2010), is the only currently known human disorder caused by a defect in an IFT protein. Do basal bodies play a more prominent role in regulating craniofacial development in humans or have the defects of axonemal proteins just not be identified yet? To elucidate this question, it will be necessary to investigate several possibilities. First, the interaction of basal body proteins with components of both Shh and Wnt pathways will have to be established. Second, we will have to determine the role of basal bodies in regulating traffic to and from the cilium. Finally, we will have to analyze the proteomes of individual cilia at various time points in development. This will ultimately provide insight into how cilia may be functioning abnormally during critical stages of development. Further, this type of analysis will be necessary across tissue types. Emerging evidence supports the notion that cilia function differently depending on cell type and on developmental stage (D'Angelo and Franco, 2010), suggesting a complexity not previously considered but potentially relevant in the context of tissue-specific phenotypes.
Conclusions
We have really just begun to understand how primary cilia function and which molecular pathways utilize these organelles. Much work remains to understand the specific function of various ciliary proteins and how different tissues use the cilia when interpreting developmental signals. It remains possible that additional pathways important in craniofacial development signal through primary cilia (e.g. Bone Morphogenic Protein (BMP)). It will be important in the future to keep cilia in mind when diagnosing craniofacial disorders with no known cause.
Fig 1.
Acknowledgements
We would like to thank Phil Beales for helpful discussion. This work was supported by the National Institute of Health (NIH) grant K99 DE019853-01 to S.A.B.
References
- Aase K, Abramsson A, Karlsson L, Betsholtz C, Eriksson U. Expression analysis of PDGF-C in adult and developing mouse tissues. Mech Dev. 2002;110:187–191. doi: 10.1016/s0925-4773(01)00560-3. [DOI] [PubMed] [Google Scholar]
- Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Current Biology. 1999;9:1304–1314. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, Ozyurek H, Farin FM, Kroes HY, Wolfrum U, Brunner HG, Cremers FP, Glass IA, Knoers NV, Roepman R. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Bachler M, Neubuser A. Expression of members of the Fgf family and their receptors during midfacial development. Mech Dev. 2001;100:313–316. doi: 10.1016/s0925-4773(00)00518-9. [DOI] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Baker K, Beales P. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet. 2009;151C:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- Balk K, Biesecker LG. The clinical atlas of Greig cephalopolysyndactyly syndrome. Am J Med Genet A. 2008;146A:548–557. doi: 10.1002/ajmg.a.32167. [DOI] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M. Neural crest inducing signals. Adv Exp Med Biol. 2006;589:24–31. doi: 10.1007/978-0-387-46954-6_2. [DOI] [PubMed] [Google Scholar]
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14:353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte S, De Angelis A, Quagliata L, Giusti F, Tammaro R, Dallai R, Ascenzi MG, Diez-Roux G, Franco B. Ofd1 is required in limb bud patterning and endochondral bone development. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Bluestone CD. Pediatric Otolaryngology. 2003;4:979–995. [Google Scholar]
- Brugmann SA, Allen NC, James AW, Mekonnen Z, Madan E, Helms JA. A primary cilia dependent etiology for midline facial disorders. Human Molecular Genetics. 2010:19. doi: 10.1093/hmg/ddq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, Ten Berge D, Fuerer C, Clevers H, Nusse R, Helms JA. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chiquet BT, Blanton SH, Burt A, Ma D, Stal S, Mulliken JB, Hecht JT. Variation in WNT genes is associated with non-syndromic cleft lip with or without cleft palate. Hum Mol Genet. 2008;17:2212–2218. doi: 10.1093/hmg/ddn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Noveen A. Phenotypic determination of epithelial appendages: genes, developmental pathways, and evolution. J Investig Dermatol Symp Proc. 1999;4:307–311. doi: 10.1038/sj.jidsp.5640235. [DOI] [PubMed] [Google Scholar]
- Cohen MM. Editorial: perspectives on craniosynostosis. American journal of medical genetics. Part A. 2005;136:313–326. doi: 10.1002/ajmg.a.30757. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Sulik KK. Perspectives on holoprosencephaly: Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J Craniofac Genet Dev Biol. 1992;12:196–244. [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortellino S, Wang C, Wang B, Bassi MR, Caretti E, Champeval D, Calmont A, Jarnik M, Burch J, Zaret KS, Larue L, Bellacosa A. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev Biol. 2009;325:225–237. doi: 10.1016/j.ydbio.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci U S A. 2004;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo A, Franco B. The primary cilium in different tissues-lessons from patients and animal models. Pediatr Nephrol. 2010 doi: 10.1007/s00467-010-1650-7. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Bertheleme JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Ruther U, Schneider-Maunoury S, Attie-Bitach T, Saunier S. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- DeMyer W. The face predicts the brain: diagnostic significance of median facial anomialies for holoprosencephaly (arhinencephay) Pediatrics August. 1964:256–263. [PubMed] [Google Scholar]
- Ding H, Wu X, Bostrom H, Kim I, Wong N, Tsoi B, O'Rourke M, Koh GY, Soriano P, Betsholtz C, Hart TC, Marazita ML, Field LL, Tam PP, Nagy A. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet. 2005;14:1109–1118. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Romio L, Castro S, Collins JE, Goulding DA, Stemple DL, Woolf AS, Wilson SW. Convergent extension movements and ciliary function are mediated by ofd1, a zebrafish orthologue of the human oral-facial-digital type 1 syndrome gene. Hum Mol Genet. 2009;18:289–303. doi: 10.1093/hmg/ddn356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Antoni L, Fu K, Whiting CJ, Francis-West P, Richman JM. Expression of WNT signalling pathway genes during chicken craniofacial development. Dev Dyn. 2009;238:1150–1165. doi: 10.1002/dvdy.21934. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Smith A. Patterning the pharyngeal arches. Bioessays. 2001;23:54–61. doi: 10.1002/1521-1878(200101)23:1<54::AID-BIES1007>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Huang B, Masyuk T, LaRusso N. Isolation of primary cilia for morphological analysis. Methods Cell Biol. 2009;94:103–115. doi: 10.1016/S0091-679X(08)94005-X. [DOI] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Ishii Y, Moriyama T, Enomoto S, Ono T, Ohyama K, Kuroda T. Seventeen-year follow-up of a patient with median cleft of the lower lip, mandible, and tongue with flexion contracture: a case report. Cleft Palate Craniofac J. 2002;39:555–559. doi: 10.1597/1545-1569_2002_039_0555_syfuoa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Brown CJ. Unravelling the complex genetics of cleft lip in the mouse model. Mamm Genome. 2001;12:426–435. doi: 10.1007/s003350010284. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Dewell SL, Brown CJ, Mager DL, Gagnier L, Mah DG. Investigations of the genomic region that contains the clf1 mutation, a causal gene in multifactorial cleft lip and palate in mice. Birth Defects Res A Clin Mol Teratol. 2005;73:103–113. doi: 10.1002/bdra.20106. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Mah DG. The clf1 gene maps to a 2- to 3-cM region of distal mouse chromosome 11. Mamm Genome. 1996;7:789. doi: 10.1007/s003359900298. [DOI] [PubMed] [Google Scholar]
- Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, MacDonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attie-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpakova-Hart E, Jinnin M, Hou B, Fukai N, Olsen BR. Kinesin-2 controls development and patterning of the vertebrate skeleton by Hedgehog- and Gli3-dependent mechanisms. Dev Biol. 2007;309:273–284. doi: 10.1016/j.ydbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou AE, Fryssira H, Sifakis S, Karadimas C, Kaminopetros P, Agrogiannis G, Velonis S, Nikkels PG, Patsouris E. Cranioectodermal dysplasia: a probable ciliopathy. Am J Med Genet A. 2009;149A:2206–2211. doi: 10.1002/ajmg.a.33013. [DOI] [PubMed] [Google Scholar]
- Lan Y, Ryan RC, Zhang Z, Bullard SA, Bush JO, Maltby KM, Lidral AC, Jiang R. Expression of Wnt9b and activation of canonical Wnt signaling during midfacial morphogenesis in mice. Dev Dyn. 2006;235:1448–1454. doi: 10.1002/dvdy.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenton KA, Nacamuli RP, Wan DC, Helms JA, Longaker MT. Cranial suture biology. Curr Top Dev Biol. 2005a;66:287–328. doi: 10.1016/S0070-2153(05)66009-7. [DOI] [PubMed] [Google Scholar]
- Lenton KA, Nacamuli RP, Wan DC, Helms JA, Longaker MT, Gerald PS. Cranial Suture Biology. Current Topics in Developmental Biology Volume. 2005b;66:287–328. doi: 10.1016/S0070-2153(05)66009-7. [DOI] [PubMed] [Google Scholar]
- Lunt SC, Haynes T, Perkins BD. Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect hedgehog signaling. Dev Dyn. 2009;238:1744–1759. doi: 10.1002/dvdy.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130:6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchac D, Renier D. Craniosynostosis. World J Surg. 1989;13:358–365. doi: 10.1007/BF01660748. [DOI] [PubMed] [Google Scholar]
- Marcucio R, Cordero DR, Hu D, Helms JA. Molecular Interactions coordinating development of the forebrain and face. Developmental Biology. 2005a;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005b;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Marshall WF. Basal bodies platforms for building cilia. Curr Top Dev Biol. 2008;85:1–22. doi: 10.1016/S0070-2153(08)00801-6. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Bronner-Fraser M. Essential role for PDGF signaling in ophthalmic trigeminal placode induction. Development. 2008;135:1863–1874. doi: 10.1242/dev.017954. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- Ming JE, Roessler E, Muenke M. Human developmental disorders and the Sonic hedgehog pathway. Mol Med Today. 1998;4:343–349. doi: 10.1016/s1357-4310(98)01299-4. [DOI] [PubMed] [Google Scholar]
- Mooney MD, Siegel MI. Understanding Craniofacial Anomalies; The etiopathogenesis of craniosynotoses and facial clefting. New York: Wiley-Liss, Inc; 2002. [Google Scholar]
- Morrison-Graham K, Schatteman GC, Bork T, Bowen-Pope DF, Weston JA. A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest-derived cells. Development. 1992;115:133–142. doi: 10.1242/dev.115.1.133. [DOI] [PubMed] [Google Scholar]
- Muenke M. Holoprosencephaly as a genetic model for normal craniofacial development. Semin Dev Biol. 1994;5:293–301. [Google Scholar]
- Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, Braun T, Casavant T, Stone EM, Sheffield VC. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci U S A. 2004;101:8664–8669. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–654. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, Carmi R, Stone EM, Sheffield VC. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101:16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, Martinelli DC, Fan CM, Peterkova R, Lesot H, Yoder BK, Sharpe PT. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 2009;136:897–903. doi: 10.1242/dev.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development. 1993;118:439–448. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Lonai P. Platelet-derived growth factor-A and its receptor are expressed in separate, but adjacent cell layers of the mouse embryo. Development. 1992;115:1045–1058. doi: 10.1242/dev.115.4.1045. [DOI] [PubMed] [Google Scholar]
- Posnick JC. Craniofacial syndromes and anomalies. In: Posnick JC, editor. Craniofacial and maxillofacial surgery in children and young adults. Philadelphia: W.B. Saunders; 2000. pp. 391–527. [Google Scholar]
- Rice D. Developmental Anatomy of Craniofacial Sutures. In: Rice D, editor. Craniofacial Sutures. Development, Disease and Treatment. Basel: Karger; 2008. [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nature Genetics. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Patel K. Wnts and the neural crest. Anat Embryol (Berl) 2005;209:349–355. doi: 10.1007/s00429-005-0459-9. [DOI] [PubMed] [Google Scholar]
- Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, Satir P, Christensen ST. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Selleck MAJ, Bronner-Fraser M. Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development (Cambridge) 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Li Y, Wang K, Wang YZ, Molotkov A, Gao L, Zhao T, Yamagami T, Wang Y, Gan Q, Pleasure DE, Zhou CJ. Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development. 2009;136:3161–3171. doi: 10.1242/dev.037440. [DOI] [PubMed] [Google Scholar]
- Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development. 2003;130:507–518. doi: 10.1242/dev.00241. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, Tada M, Mayor R, Burns AJ, Lupski JR, Hammond P, Beales PL. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung's disease in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2008;105:6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes and Development. 1999;13:3136–3148. doi: 10.1101/gad.13.23.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Gessler M, Grzeschik KH. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539–540. doi: 10.1038/352539a0. [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, Szczepanska M, Krawczynski M, Zachwieja J, Zwolinska D, Beales PL, Ropers HH, Latos-Bielenska A, Kuss AW. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 2010;86:949–956. doi: 10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shackleford GM. Murine Wnt10a and Wnt10b: cloning and expression in developing limbs, face and skin of embryos and in adults. Oncogene. 1996;13:1537–1544. [PubMed] [Google Scholar]
- Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet. 2009;18:4565–4575. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Chong HJ, Hu D, Hallgrimsson B, Marcucio RS. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development. 2010;137:3405–3409. doi: 10.1242/dev.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Functional modules, mutational load and human genetic disease. Trends Genet. 2010;26:168–176. doi: 10.1016/j.tig.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KW. Beitrage zur Kenntnis einiger Drusen und Epithelien. Arch. mikr. Anat. u Entwick. 1898;52:552. [Google Scholar]