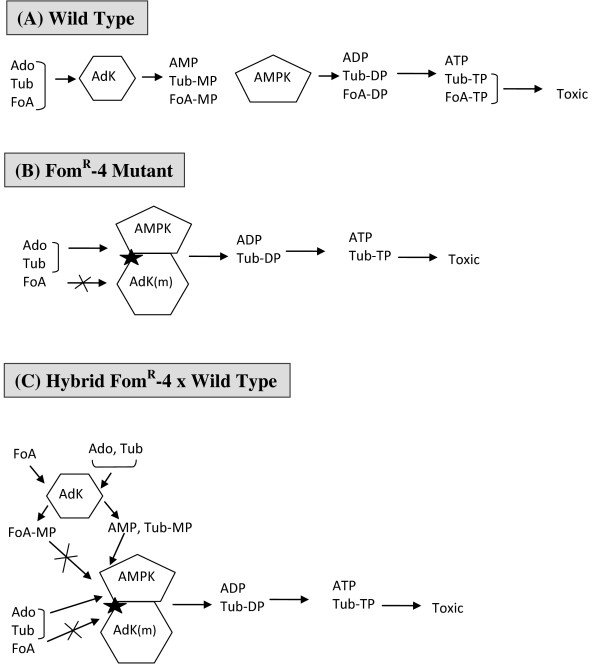
Figure 7.
A model to account for the lack of AdK activity in the cell extracts of the FomR-4 mutant and dominant expression of its drug-resistance phenotype. (A) In the WT cells, AdK converts Ado and various Ado-analogs (e.g. FoA, Tub) into their corresponding monophosphates; subsequently AMP-kinase (AMPK) and other enzymes convert them into di- and tri-phosphates. (B) and (C), In the FomR-4 mutant or the cell hybrids formed between FomR-4 and the WT cells, the Ser191Phe mutation in AdK (indicated by ✭) leads to its complex formation with AMPK. This mutation is also postulated to specifically prevent the binding of FoA and FoA-MP to the AdK-AMPK complex. As a result of this complex formation, AMP (or Tub-MP) formed by AdK is not released but directly transferred to the AMPK for conversion into ADP. These account for the unusual properties of the FomR-4 mutant.