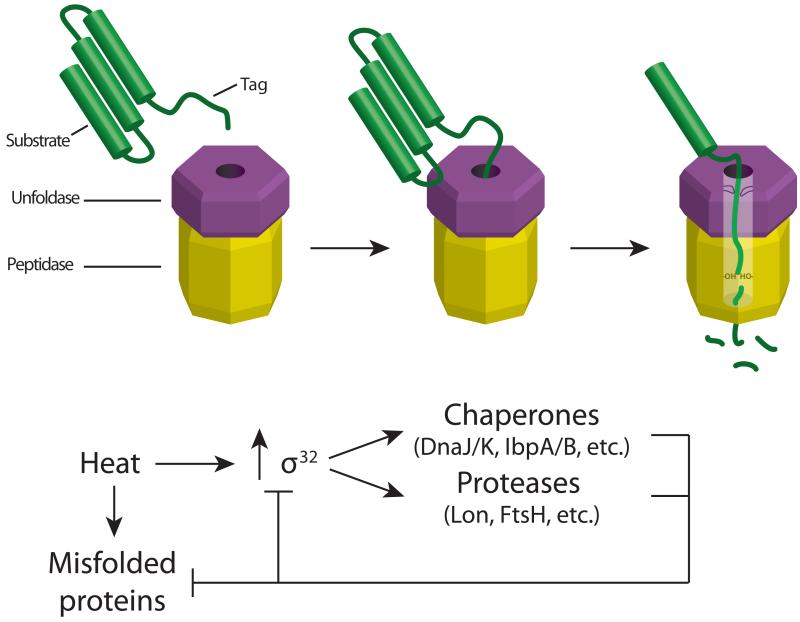
A. Recognition, unfolding, and degradation of substrates by a AAA+ protease. AAA+ proteases consist of a hexameric AAA+ unfoldase ring stacked on top of a peptidase chamber. Substrates are often initially recognized through binding of exposed peptide degradation tags to loops located within the central pore of the AAA+ unfoldase ring. Cycles of ATP binding and hydrolysis by the AAA+ ring result in unfolding of the bound substrate during translocation into the proteolytic chamber. Protease active sites then degrade the substrate into small peptide fragments that are released.
B. Regulation of σ32 in E. coli. Elevated temperatures lead to misfolded proteins as well as increased σ32 activity, activating the heat shock response. Chaperones and proteases are among the upregulated members of the heat shock regulon, and their activity serves both to alleviate misfolded proteins and to downregulate σ32 activity, thereby limiting the heat shock response.