Table 2.
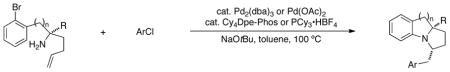
Cascade Intramolecular N-Arylation/Intermolecular Carboamination Reactions
 | |||||
|---|---|---|---|---|---|
| entry | substrate | product | catalystb | drc | yield (%)d |
| 1 |
|
|
A | >25:1 (20:1) | 76 |
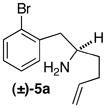
| 2 | (±)-5a |
|
A | 8:1 (5:1) | 78 |
| 3 | (±)-5a |
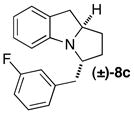
|
Be | >25:1 (5:1) | 42 |
| 4 |
(+)-5a 92% ee |
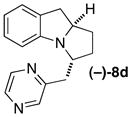
|
B | 8:1 (7:1) 86% ee |
58 |
| 5 |
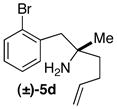
|
|
B | 14:1 (5:1) | 60 |
| 6 |
(−)-5d 87% ee |
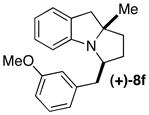
|
A | >25:1 (10:1) 91% ee |
75 |
| 7 |
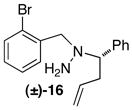
|
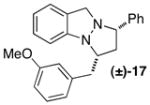
|
C | 15:1 (3:1) | 45 |
| 8 |
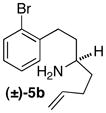
|
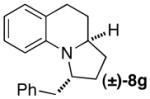
|
A | 25:1 (12:1) | 68 |
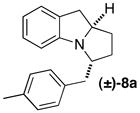
| 9 | (±)-5b |
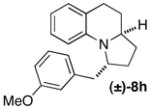
|
A | 22:1 (8:1) | 63 |
| 10 | (±)-5b |
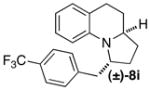
|
Be | 7:1 (3:1) | 56 |
| 11 |
(+)-5b 88% ee |
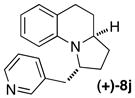
|
B | >25:1 (10:1) 92% ee |
56 |
| 12 |
|
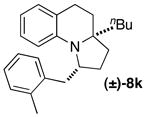
|
B | 11:1 (12:1) | 79 |
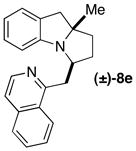
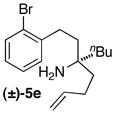
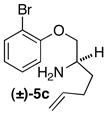
| 13 | (±)-5e |
|
B | 10:1 (7:1) | 47 |
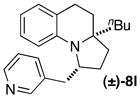
| 14 |
|
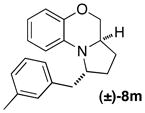
|
A | 15:1 (5:1) | 35 |
Conditions: Reactions were conducted on a 0.25 mmol scale using 1.0 equiv of substrate, 1.2 equiv of ArCl, 2.4 equiv of NaOtBu, catalyst (4 mol % [Pd]), toluene (0.25 M), 100 °C.
Catalyst A: Pd(OAc)2 (4 mol %), Cy4Dpe-Phos 15 (4 mol %). Catalyst B: Pd2(dba)3 (2 mol % complex), PCy3•HBF4 (4 mol %). Catalyst C: Pd2(dba)3 (2 mol % complex), X-Phos 14 (8 mol %).
Diastereomeric ratios are reported for the isolated products. Diastereomeric ratios in parentheses were observed in crude reaction mixtures.
Isolated yield (average of two experiments).
The reaction was conducted using 8 mol % PCy3•HBF4.
