Abstract
The success of allogeneic hematopoietic cell transplantation (HCT) for B-cell malignancies is evidence that these tumors can be eliminated by T lymphocytes. This has encouraged the development of specific adoptive T-cell therapy, both for augmenting the anti-tumor effect of HCT and for patients not undergoing HCT. T cells that are capable of recognizing antigens expressed on malignant B cells may be recruited from the endogenous repertoire or engineered to express tumor-targeting receptors. Critical insights into the qualities of T cells that enable their persistence and function in vivo have been derived, and obstacles to effective T-cell-mediated tumor eradication are being elucidated. These advances provide the tools to translate adoptive T-cell transfer into reliable clinical therapies.
Keywords: adoptive T-cell therapy, allogeneic hematopoietic cell transplantation, central memory T cell, chimeric antigen receptor, immunoglobulin idiotype, graft-versus-host disease, graft-versus-tumor effect, minor histocompatibility antigen, T-cell receptor, tumor antigen
The potential to use immune-based therapies for human malignancies is attractive because of the specificity of antibody and T-cell recognition. The most profound advances have been made in the development of antibodies as therapeutics, and several monoclonal antibodies that recognize molecules on the surface of cancer cells are being used in human cancer therapy. Antibodies that target CD20 and CD52 are now routinely employed in standard therapeutic regimens for subsets of patients with B-cell malignancies [1–3]. The development of effective T-cell therapy for human malignancy either through vaccination or by adoptive T-cell transfer, which refers to the isolation, expansion and reinfusion of tumor-reactive T cells, has been substantially more challenging. The difficulties in developing T-cell-based immunotherapies are due, in part, to the inability of current vaccines to reproducibly elicit effective tumor-reactive T-cell responses, and the complexity of deriving and expanding tumor-reactive T cells ex vivo that have the capacity to persist and function following adoptive transfer. Despite these obstacles, the exquisite ability of T cells to distinguish diseased from normal cells has encouraged the continued investigation of strategies to employ T cells as therapeutic agents.
There is evidence from allogeneic hematopoietic cell transplantation (HCT) that advanced B-cell malignancies are susceptible to a T-cell-mediated graft-versus-tumor (GVT) effect, although GVT activity cannot yet be reproducibly separated from graft-versus-host disease (GVHD) [4–7]. The demonstration that B-cell tumors are recognized by T cells has provided optimism that donor T cells specific for tumor-associated antigens might be isolated, expanded and administered to the patient to augment the GVT effect; or that autologous T cells might be elicited or engineered to recognize tumor-associated antigens, without the need for allogeneic HCT. Engineering of tumor reactive T cells can be accomplished by gene transfer techniques that introduce a T-cell receptor (TCR) with specificity for peptide fragments of intracellular proteins displayed on class I and class II MHC molecules expressed by the tumor, or a chimeric antigen receptor (CAR) that consists of a single-chain antibody fragment (scFv) specific for a B-cell surface molecule linked to the ζ-chain of the CD3/TCR complex [8,9]. This review will discuss the rationale and theoretical framework for developing adoptive T-cell therapy for B-cell malignancies, the obstacles that have been encountered, and the directions that are currently being taken for clinical translation.
GVT effect of allogeneic HCT in B-cell malignancies
Allogeneic HCT provides a potentially curative therapy for a variety of hematologic malignancies, including many B-cell tumors. Originally, allogeneic HCT was developed as a method of rescuing patients from the lethal toxicity of high doses of myeloablative chemoradiotherapy administered to achieve greater tumor-cell killing than could be achieved with conventional doses of chemotherapy [10], and was used as a treatment of last resort for patients with refractory leukemia, including B-lineage acute lymphoblastic leukemia (B-ALL). Consistent with the prediction from murine models, that immune recognition of tumor cells could contribute to tumor eradication, human allogeneic HCT for B-ALL was accompanied by a T-cell mediated GVT effect [11–13]. A GVT effect of allogeneic HCT has subsequently been confirmed in other B-cell malignancies, including chronic lymphocytic leukemia (B-CLL), multiple myeloma (MM) and non-Hodgkin lymphoma (NHL) [14–18]. The myeloablative chemotherapy regimen contributes substantially to tumor control but it is now realized that the curative potential of this procedure is a result of the immunologic elimination of malignant cells. This is evident from the strikingly lower relapse rate and increased leukemia-free survival rate in patients receiving an allogeneic HCT compared with syngeneic HCT [12,13]. There are several factors that limit the success of allogeneic HCT and efforts to improve outcome are focused on reducing toxicity due to conditioning and GVHD, and augmenting the GVT effect (Table 1).
Table 1.
Factors that limit the success of allogeneic hematopoietic cell transplantation.
| Obstacle | Strategy |
|---|---|
| Toxicity due to the conditioning regimen | Employ reduced-intensity conditioning regimens |
| Graft-versus-host disease | Improve drug regimens for immunosuppression Targeted suppression of alloreactive T-cell activation or function Remove alloreactive T cells from the donor stem cell graft |
| Tumor relapse | Adoptive transfer of tumor-reactive T cells Vaccination to elicit tumor-reactive T-cell responses in vivo |
Donor lymphocyte infusions for B-cell malignancies
A critical role for donor T cells in the GVT effect was demonstrated by studies by Kolb et al. who investigated the use of donor lymphocyte infusions (DLIs) in patients with leukemia relapse after allogeneic HCT [19]. Durable complete remissions were achieved in 10–40% of patients with B-ALL, B-CLL, MM and lymphomas [20–22]. This compares with response rates of up to 70% in patients with relapsed chronic myeloid leukemia (CML) and it has been hypothesized that the superior ability of CML cells to differentiate into antigen presenting dendritic cells (DCs) and prime anti-tumor T-cell responses may be responsible for these differences in outcome [19,23]. To improve the outcome of patients with B-cell malignancies receiving DLIs, strategies such as the administration of pre-DLI chemotherapy or the use of ex vivo activated donor lymphocytes are being explored. The induction of GVHD remains a major complication associated with the use of donor lymphocytes. Although some patients receiving DLI achieve complete remissions in the absence of clinically evident GVHD, most responding patients develop acute and chronic GVHD suggesting the antigens that are recognized on tumor cells are often shared with other tissues. The risk of GVHD can be attenuated in a subset of patients by using gradually escalating doses of DLI but this strategy is most effectively applied in slowly progressive malignancies [24–26].
Reduced-intensity conditioning regimens for allogeneic HCT
Allogeneic HCT employing myeloablative conditioning is restricted to younger and medically fit patients to avoid excessive mortality from toxicities related to chemoradiotherapy. The median age at diagnosis for B-CLL and MM is over 60 years and these patients often have comorbidities due to their age, underlying malignancy or prior chemotherapy. This limitation to the use of allogeneic HCT was overcome by the development of reduced-intensity conditioning (RIC) regimens that use low doses of chemoradiotherapy to immunosuppress the recipient sufficiently to prevent rejection of the donor stem and T-cell graft, and enable a GVT effect to mediate tumor eradication [27,28]. RIC-HCT has been effective in several indolent B-cell malignancies, including B-CLL, MM and lymphoma, although aggressive lymphoma and B-ALL are less responsive [5–7,29]. Similar to the observations made in patients receiving myeloablative HCT, the anti-tumor efficacy of RIC-HCT is highly correlated with GVHD, which often requires long-term immunosuppressive therapy and is a major cause of morbidity and mortality. Therefore, the development of approaches that could be incorporated with HCT, such as the adoptive transfer of T cells that can specifically target antigens that are preferentially or selectively expressed on the tumor to augment the GVT effect without inducing GVHD, is a high priority of current research efforts.
Minor histocompatibility antigens as targets for the GVT effect
In the context of an allogeneic HCT from an HLA-identical donor, T-cell recognition of minor histocompatibility antigens (minor H antigens) is responsible for GVHD and has been implicated in the GVT effect. Minor H antigens result from genetic polymorphisms between the HCT recipient and the corresponding HLA-identical stem cell donor [30]. The most common mechanism for generating a minor H antigen is a non-synonymous single nucleotide polymorphism (SNP) that results in a peptide presented by HLA-molecules on recipient cells to which the HLA-identical donor is not tolerant and can be recognized as ‘non-self’ by donor CD8+ and CD4+ T cells [31]. Other mechanisms for generating minor H antigens including alternative splicing of peptide fragments, and differential protein expression as a consequence of gene deletion have also been identified [32,33].
Reduced-intensity conditioning-HCT relies almost exclusively on immune elimination of tumor cells and provides an opportunity to dissect the specificity of tumor-reactive T cells that develop after HCT, and identify minor H or tumor-associated antigens that are the targets of the GVT response. In a study at the Fred Hutchinson Cancer Research Center (FHCRC) we analyzed the temporal kinetics and specificity of T cells that develop after non-myeloablative-HCT for B-CLL because large numbers of tumor cells could be stored pre-transplant and used to assess recognition by donor T cells that developed in the recipient post-transplant [34]. We found that CD8+ and CD4+ T-cell responses directed against minor H antigens expressed by B-CLL developed in all patients that achieved sustained tumor regression and correlated with the clearance of tumor cells from peripheral blood, bone marrow and lymph nodes. By contrast, patients with progressive B-CLL after HCT failed to develop T cells that recognized leukemic cells, even though they often developed GVHD, suggesting the failure to eradicate the tumor in these patients was because alloreactive T cells only recognized antigens that were not shared by target tissues of GVHD and the tumor (Figure 1) [34]. The kinetics with which tumor-reactive T cells developed in responding patients after RIC-HCT varied from several weeks to 1 year, however once established, these T cells persisted long-term, indicating that immunologic memory was established. Analysis of the specificity of CD8+ T cells that recognized B-CLL in patients that achieved a complete remission demonstrated that multiple minor H antigens were being targeted, including those that were broadly expressed on both leukemic cells and nonhematopoietic tissues [34].
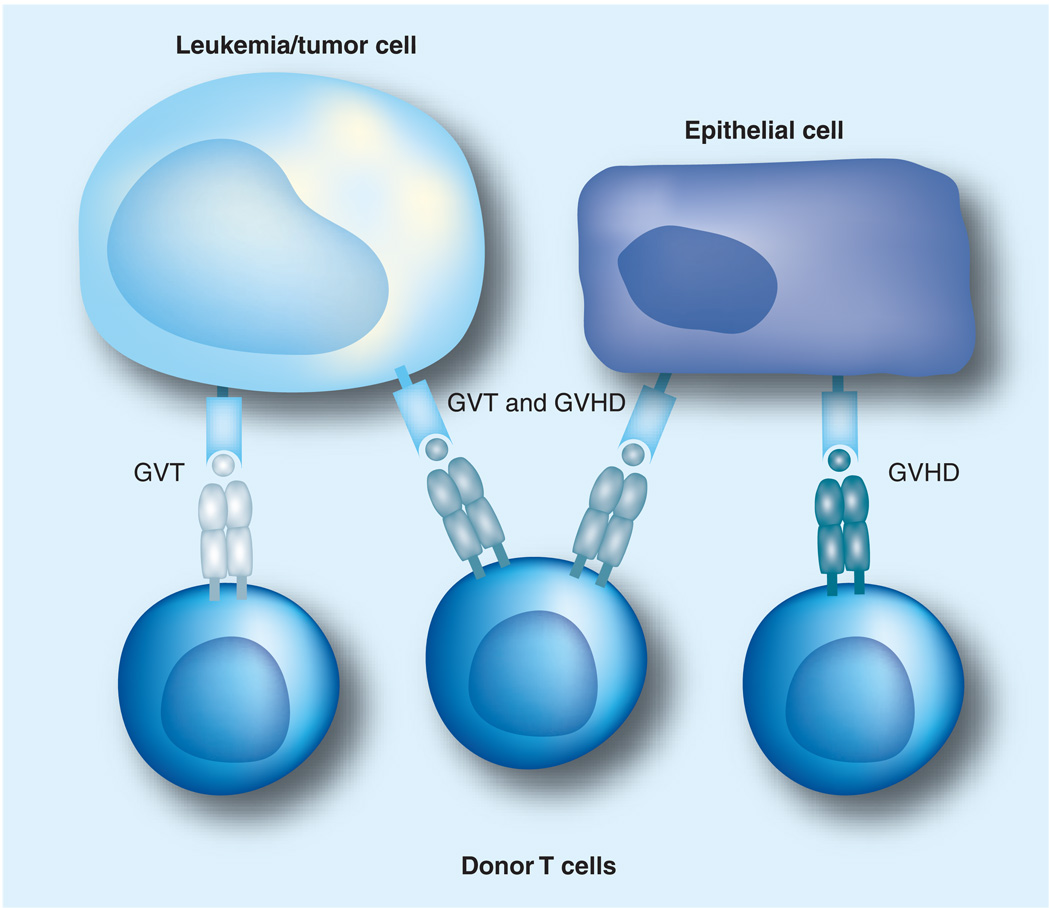
Figure 1. Expression of minor H antigens on tumor cells and epithelium dictate GVHD and GVT activity mediated by donor T cells.
Minor H antigens that are selectively expressed on tumor cells but not on epithelium can serve as targets for a selective GVT response in the absence of GVHD. T cells specific for minor H antigens expressed on both tumor and epithelium can mediate both a GVT effect and GVHD, those specific for minor H antigens on tumor but not epithelium mediate a selective GVT response, while those specific for minor H antigens on epithelium but not on tumor only mediate GVHD.
GVHD: Graft-versus-host disease; GVT: Graft-versus-tumor.
A focus of current work in the field of allogeneic HCT is the identification of minor H antigens that are limited in their expression to normal and malignant hematopoietic cells, and are absent on GVHD target tissues. Several minor H antigens that meet this prerequisite have been discovered, including HA-1, ACC-1 and LRH-1 [31,35,36], which are selectively expressed on all hematopoietic cells; and PANE-1, HB-1 and CD19, which are exclusively expressed on B-lineage cells and could be used to target B-cell malignancies [37–39]. However, the utility of an individual hematopoietic-restricted minor H antigen as a target for T-cell therapy is determined by the frequency of the HLA allele that presents the antigen and the requirement that the patient and donor be mismatched for the minor H antigen, in the appropriate direction. For HA-1, the most extensively studied minor H antigen, the disparity rate is estimated to be 4.2–8.5% in HLA-A2+ sibling pairs and 6.8–12.2% in HLA-A2 matched unrelated pairs [40]. These data illustrate a problem for the translation of minor H antigen specific T-cell therapy into clinical practice with the panel of minor H antigens that are presently known, since only a few patients could actually benefit from this strategy. This obstacle will only be resolved by the discovery of additional minor H antigens. The HapMap project has identified over 104 SNPs in the human genome that confer amino acid sequence changes in coding regions and this database has enabled the use of genome association studies for minor H antigen discovery, which should rapidly expand the number of minor H antigens at our disposal [41]. A second obstacle for targeting minor H antigens is the need to derive and expand minor H antigen-specific T cells from the donor for adoptive transfer in every case. A potential solution discussed later in this review is the isolation of TCR genes from high-avidity minor H antigen-specific T cells, construction of gene transfer vectors and engineering of primary donor T cells for transfer in suitable patients.
Adoptive immunotherapy with T cells specific for nonpolymorphic tumor-associated antigens
Ideally, nonpolymorphic antigens expressed on B-cell tumors could be targeted using autologous T cells to circumvent the need to find a suitable donor for allogeneic HCT and the complications associated with HCT. In our analysis of T-cell responses that developed after allogeneic RIC-HCT for B-CLL, we were surprised to find that a proportion of T-cell clones were specific for nonshared and shared determinants that are only expressed on the recipient B-CLL, and not on Epstein–Barr virus (EBV)-transformed B cells [34]. Additional studies are in progress to characterize these putative tumor-specific antigens that are targeted as part of the anti-B-CLL response after transplant, and to determine whether they represent tumor-specific mutations or nonpolymorphic proteins. Several candidate tumor-specific proteins have already been identified in distinct subtypes of B-cell malignancies and are being pursued as targets for T-cell therapy. These include the immunoglobulin idiotype (Id), the bcr–abl protein in Philadelphia chromosome-positive B-ALL [42], and EBV-associated proteins that are expressed in EBV-positive Hodgkin disease (HD), Burkitt’s lymphoma (BL), and EBV-induced lymphoproliferations that develop in severely immunocompromised patients. In addition to tumor-specific determinants that are restricted in their expression to the B-cell tumor, many proteins have been found to be overexpressed or aberrantly expressed in malignant B cells and to be present at much lower levels in normal tissues (Table 2). Tolerance to many of these tumor-associated self-proteins is not complete and it has been possible to isolate T cells that selectively lyse tumor cells. Whether such T cells can be used for adoptive therapy without excessive damage to normal tissues will require evaluation in pilot clinical trials.
Table 2.
Nonpolymorphic antigens in B-cell malignancies.
| Antigen class | Example | Tumor expression | Ref. |
|---|---|---|---|
| Immunoglobulin idiotype | CDR region of Id | Individual Id expressed in each B-cell malignancy | [51] |
| Viral antigens | EBV latent proteins (e.g., EBNA-1, 3, LMP-1,2) | EBV-LPD, Hodgkin disease | [65,68] |
| Chromosome translocation | Bcr/abl | Philadelphia chromosome-positive B-ALL | [42] |
| Overexpressed proteins | Bcl-2 | B-CLL | [149] |
| Fibromodulin | B-CLL | [74] | |
| Mdm2 | B-CLL | [73] | |
| RHAMM/CD168 | B-CLL | [150] | |
| Survivin | B-CLL | [75] | |
| DKK1 | MM | [78] | |
| HM1.24 | MM | [77] | |
| PRAME | MM, B-CLL | [150] | |
| SPAN-XB | MM | [76] | |
| WT-1 | MM, B-ALL | [80,81] | |
| Aberrantly expressed proteins | Cancer-testes antigens (e.g., NY-ESO-1, LAGE, MAGE) | MM | [80] |
B-ALL: B-cell acute lymphoblastic leukemia; B-CLL: B-cell chronic lymphocytic leukemia; CDR: Complementarity determining region; EBV: Epstein–Barr virus; Id: Idiotype; LPD: Lymphoproliferative disease; MM: Multiple myeloma.
Tumor-specific antigens: targeting the immunoglobulin idiotype
B-cell malignancies derive from a single B-cell clone that expresses a unique immunoglobulin (Ig) molecule on the cell surface. The variable regions of the heavy and light chains of the tumor Ig contain determinants that are specific for the malignant clone and are referred to as Id. The Id is a specific target for immunotherapy and passive immunotherapy with custom-made monoclonal anti-Id antibodies inducing an anti-tumor effect in up to 66% of patients, and long-lasting tumor regressions achieved in some cases [43]. However, this approach failed to completely eradicate all malignant B cells, and some patients relapsed owing to out-growth of a mutated tumor Id-variant that had lost the epitope recognized by the monoclonal antibody [43,44]. T-cell recognition of Id-derived epitopes has also been demonstrated, and Id-specific T cells were shown to eradicate B-cell tumors in murine myeloma models [45].
Based on the partial efficacy of passive antibody therapy in humans and animal model data demonstrating anti-tumor activity of Id-specific T cells, it was logical to evaluate vaccination regimens that might elicit both antibody and T-cell responses. Clinical trials have focused on patients with follicular lymphoma (FL), which is sufficiently indolent to provide time to produce the vaccine and to develop an immune response after vaccination. Studies by Kwak et al. showed that immunization with autologous Id protein generated Id-specific humoral and cellular immune responses in patients with FL [46]. In subsequent trials, adjuvant cytokines and Id-loaded DCs were incorporated into vaccine regimens in an effort to improve immunogenicity and efficacy [47,48]. In a Phase I/II study by Bendandi et al., in which granulocyte macrophage colony-stimulating factor was added to the vaccine, anti-Id antibody responses were induced in 15 out of 20 patients and CD8+/CD4+ T-cell responses were induced in 19 out of 20 patients. A subset of these patients achieved sustained molecular remissions of their lymphoma, providing evidence for an anti-tumor effect of vaccination [48]. A subsequent Phase II study in patients who had relapsed after initial chemotherapy, confirmed the immunogenicity of this approach and suggested a clinical benefit of vaccination based on a prolonged duration of complete response to reinduction chemotherapy [49]. Both humoral and cellular immune responses may be important in anti-tumor effects following anti-Id vaccination, although a fraction of patients who achieved complete remission after Id vaccination did so in the absence of detectable antibody responses [48]. A recent retrospective analysis of FL patients who had received Id vaccinations addressed the question whether the superior clinical outcome associated with an anti-Id immune response also resulted in an improved overall survival (OS). The study showed that the generation of an antibody response correlated with an improved OS at 10 years, whereas the generation of an anti-Id T-cell response was surprisingly not associated with improved OS [50]. This could reflect the sensitivity of assays to detect Id-reactive T cells compared with those to evaluate humoral immunity. Characterization of anti-Id T-cell reactivity has mapped the immunogenic epitopes within the hypervariable complementarity determining regions of the Ig heavy chain [51]. Of interest, in vitro studies have also suggested that Ig framework-derived peptides can function as cytotoxic T-cell epitopes [52], although mapping of the specificity of responses in vaccinated patients has yet to reveal recognition of framework regions.
The potential benefit of Id vaccination in FL has now been investigated in three Phase III studies [53]. The results of these trials have not yet been published but preliminary information that has been released indicates that only one trial showed a significant benefit in disease-free survival for vaccinated patients [53]. This study included 177 previously untreated patients with FL who achieved a complete remission after induction chemotherapy. The median disease-free survival for patients who received Id vaccination was 33.8 months versus 21.1 months in the control arm. This advantage in disease-free survival has not been observed in the two other studies [53]. The publication of data from these trials is awaited with interest, and may provide insight into what factors contribute to the differences in outcome following Id vaccination.
Compared with the success of Id vaccination in FL, the induction of anti-Id immunity in MM and aggressive lymphoma has been more challenging, and clinical responses have only been observed in a minority of patients [54,55]. The neutralization of anti-Id-antibodies by circulating myeloma protein and immune-escape mechanisms, such as the secretion of TGF-β and IL-10, by myeloma cells may compromise the priming and/or function of anti-Id T-cell responses [56]. Additionally, studies in murine myeloma models showed that Id-specific T cells are progressively deleted from the T-cell repertoire with increasing serum levels of myeloma protein [56,57]. Even if reactive T cells can be elicited, the functional phenotype may be critical in anti-tumor activity, with cytotoxic T lymphocytes and Th1 cells inhibiting tumor growth and Th2 cells promoting tumor progression [58].
Idiotype-specific T cells can be generated in vitro through stimulation with peptide-pulsed or Id-transduced autologous DCs, as well as through genetic introduction of chimeric anti-Id receptors, but efforts to employ adoptive T-cell transfer to target Ids have been limited to animal model studies [59,60]. The requirement to produce a customized Id product from each patient, to use as a reagent for antigen presentation, combined with the complexity of reproducibly deriving a T-cell product for adoptive therapy from each patient makes such an effort challenging. The use of vaccination with Id-protein or Id-loaded DC to elicit Id-specific T cells that could then be more readily isolated and expanded ex vivo may provide a solution to the latter problem, and facilitate studies to determine if vaccination combined with adoptive T-cell transfer could improve upon the results achieved with vaccination alone [55]. Furthermore, vaccination with tumor lysate-loaded DCs may represent a strategy to induce or reactivate a T-cell response with reactivity against multiple tumor antigens, including the Ig–Id [61].
Targeting EBV-associated B-cell malignancies with T-cell therapy
The lymphotropic EBV was first discovered in cultured lymphocytes from BL [62], and has also been detected in HD. The oncogenic potential of EBV is evident in severely immunocompromised HCT and solid-organ transplant patients who develop EBV-driven B-cell proliferations, termed EBV-associated lymphoproliferative disease. These tumors develop as a consequence of inadequate T-cell surveillance, and express the full range of latent cycle EBV antigens, including Epstein–Barr nuclear antigen (EBNA)-3A, -3B and -3C proteins, which serve as targets for T cells in immunocompetent donors. Indeed, EBV-associated lymphoproliferative disease that occurred in recipients of T-cell depleted allogeneic HCT can be successfully treated by the adoptive transfer of unselected lymphocytes from the respective EBV-seropositive donor, although this approach is complicated by GVHD [63]. To avoid GVHD, Rooney et al. isolated EBV-specific T cells from the peripheral blood of HCT donors by repeated in vitro stimulation and demonstrated that adoptive transfer of only the EBV-reactive T cells promoted regression of established EBV-driven lymphomas, and prevented the development of tumors when administered prophylactically [64]. The introduction of a marker gene into a subset of transferred EBV-specific T cells was used to track infused T cells and demonstrated that the cells persisted long-term in vivo [64].
Epstein–Barr virus is also associated with BL and HD that occur in immunocompetent individuals, but these tumors express a very restricted set of EBV antigens, that presumably facilitates their escape from host immunity. EBNA-1 is the only EBV protein expressed in BL but is not recognized efficiently by CD8+ T cells [65,66]. HD also expresses EBNA-1, and the weakly immunogenic latent membrane proteins (LMP-1) and -2. Although LMP-1 and -2 are weakly immunogenic, strategies for isolating and expanding autologous T cells specific for these antigens from patients with HD have been developed [67,68]. Pilot studies in which T cells targeting these EBV antigens have been expanded and adoptively transferred to patients with HD have shown that the EBV-reactive T cells migrate to tumor sites and result in tumor regression in a subset of patients [69].
Targeting nonmutated tumor-associated antigens derived from self-proteins
Microarray studies that have compared the gene-expression profile of malignant B cells to their respective normal counterparts have uncovered highly expressed genes in the various histologic types of B-cell tumors [70–72]. Algorithms that predict peptide binding to MHC have identified potentially immunogenic peptide epitopes that might serve as targets for T-cell therapy in several overexpressed proteins, including MDM2, fibromodulin and survivin in B-CLL [73–75]; NY-ESO-1, SPAN-XB, DKK1, HM1.24 and WT-1 in MM [76–80] and WT-1 in B-ALL [81]. Tumor-reactive CD8+ T cells that recognize these and other self-peptides have been isolated from normal donors using in vitro culture techniques [73–78,82,83]. However, it is often difficult to isolate T cells with high affinity to these self-antigens from patients who have been pretreated with cytotoxic chemotherapy that affects T-lymphocyte numbers and function. Additionally, it remains to be determined whether targeting antigens that are also expressed in some normal tissues with T-cell therapy may lead to undesired toxicity.
Efforts to target self-proteins expressed on B-cell tumors by adoptive therapy have initially focused on WT-1 and NY-ESO-1, which are only expressed at low levels in very few normal tissues but are highly expressed in tumor cells of some patients. WT-1 is expressed at high levels in myeloid leukemias and B-ALL and at very low levels on CD34+ hematopoietic stem cells. WT-1-specific T-cell responses have been elicited in normal donors and implicated in the GVT effect against B-ALL after allogeneic HCT [83,84]. A WT-1 peptide vaccine increased the frequency of WT-1-tetramer-positive T cells and induced an anti-tumor response in a subset of leukemia patients without adverse events [85]. Techniques for the isolation of T cells specific for multiple epitopes in WT-1 from normal donors have been described [82,83], and clinical trials of adoptive T-cell therapy targeting WT-1 have been initiated.
NY-ESO-1 is a member of the cancer testis (CT) family of proteins that are normally only present in the testis and the placenta, but are upregulated in a variety of tumor types, including a subset of patients with MM and melanoma. NY-ESO-1 was found to be the most immunogenic CT antigen and both spontaneous and vaccine-induced immune responses against NY-ESO-1 have been reported in cancer patients [86,87]. A clinical trial of adoptive T-cell therapy for melanoma with autologous CD4+ NY-ESO-1-specific T-cell clones resulted in a remission in one out of nine patients, illustrating the potential for T cells of this specificity to mediate anti-tumor activity [88]. These results have implications for the development of adoptive T-cell therapy for MM, since many members of the CT antigen family, including NY-ESO-1, are expressed in myeloma cells and these proteins have been implicated in tumor progression [89].
Genetic modification: endowing T cells with receptors that target tumor antigens
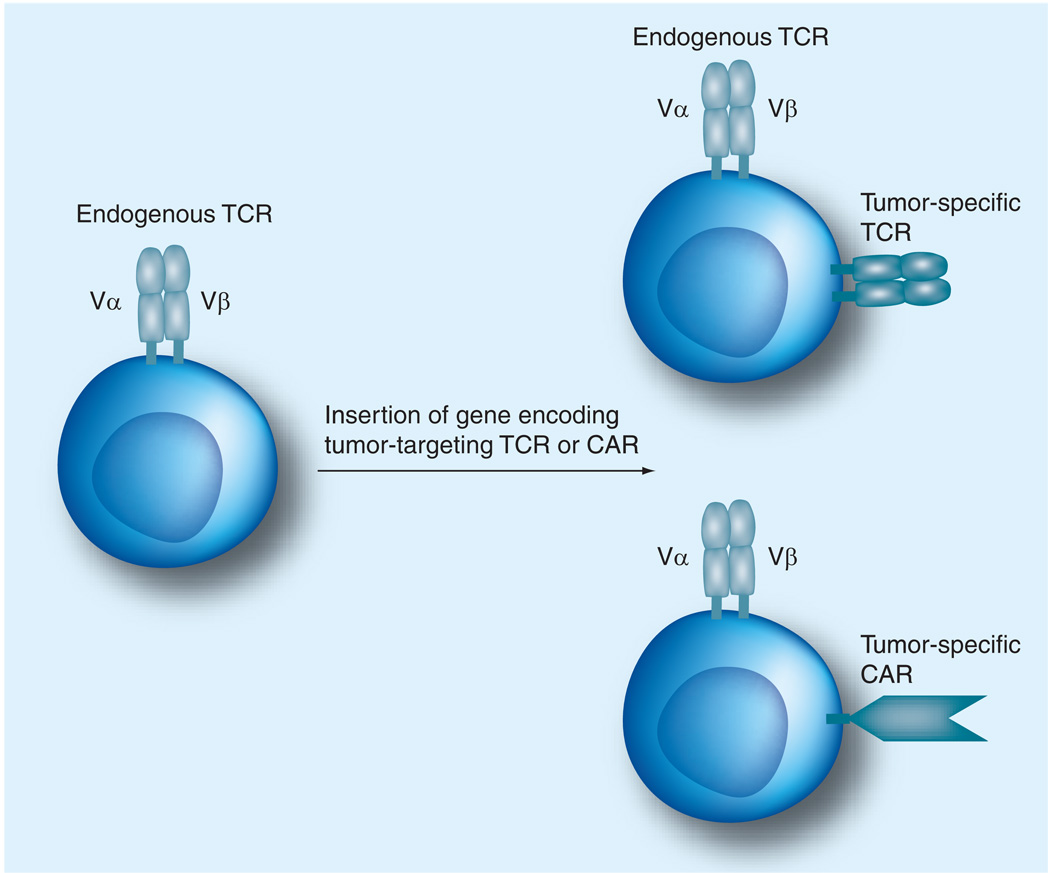
Although polymorphic and tumor-associated antigens have been identified for B-cell malignancies and mediate anti-tumor activity in vitro or in preclinical models, their translation into clinical trials is hampered in many cases by the failure to reproducibly isolate high-affinity T cells from patients or healthy donors that are specific for these antigens. The promise of adoptive T-cell therapy will only be realized if methods for the reproducible production of tumor-reactive T cells for therapy are developed. The genetic modification of T cells provides a potential solution for this problem, since T cells can be engineered to express a TCR capable of recognizing the desired antigen with high affinity or to express an artificial CAR that is specific for a tumor cell surface molecule (Figure 2).
Figure 2. Gene transfer can retarget primary T cells to recognize tumor cells.
Schematic demonstration of the engineering of bispecific T cells by inserting the α- and β-genes of a TCR specific for a tumor-associated antigen, or a gene encoding a CAR constructed of a single-chain antibody fragment fused to TCR signaling domains.
CAR: Chimeric antigen receptor; TCR: T-cell receptor.
T-cell receptor gene transfer
The specificity of T-cell recognition is provided by the TCR, and the transfer of TCR-α and -β genes into recipient T cells can endow the cells with the antigen specificity of the introduced TCR [90]. Thus, one approach to derive T cells for therapy of B-cell tumors is to introduce TCR genes from clones with specificity for a viral, minor H antigen, or nonpolymorphic tumor antigen into T lymphocytes of a patient [8,91–94]. The TCR-α and -β genes can be derived from the rare T cells that have been isolated with high affinity for the desired antigen, or from transgenic mice that express human HLA alleles and have been immunized with the human protein to generate a high-affinity murine T-cell response from which the murine HLA-restricted TCRs can be cloned [93].
Gene transfer into T cells
The majority of TCR transfer studies have employed γ-retroviral vectors (RV) based on murine leukemia virus or murine sarcoma virus, or self-inactivating lentiviral vectors (LVs) [95,96]. A characteristic of RV is that cell division is required for vector integration following infection [97]. By contrast, LVs have the ability to transduce a variety of slowly or even nondividing cells, including unstimulated T cells in the absence of TCR activation [98]. For the genetic modification of T cells, this may be an advantage since TCR activation and sustained proliferation required for RV transduction may impair the ability of transduced T cells to persist after adoptive transfer and reduce their anti-tumor activity. Both RV and LV preferentially integrate into transcriptionally active genes, and entail a risk of insertional mutagenesis and transformation of modified T cells, although LVs are considered to be less genotoxic [99,100]. The development of lymphoproliferative complications due to dysregulated proto-oncogene expression in a gene therapy trial that used engineered hematopoietic stem cells in patients with X-linked severe combined immunodeficiency (SCID), highlighted the risk related to cell transformation as a consequence of gene insertion and has led to the development of self-inactivating vectors with reduced mutagenic potential (Box 1) [101–103]. Compared with hematopoietic stem cells, mature T cells have been found to be more resistant to oncogene transformation and so far, no case of malignant transformation has been reported in adoptive immunotherapy trials that employed T cells genetically modified with suicide genes or gene markers [104]. Nonviral gene-delivery systems, such as electroporation into T cells have also been developed, however, the expression that can be achieved with plasmid DNA may vary considerably among T cells and is only transient after electroporation of RNA [105,106]. A potentially attractive nonviral system for stable gene transfer is the sleeping beauty (SB) transposon technology. SB is a synthetic transposable element that has been generated from the ancestral Tc1/mariner-like transposon in fish [107]. DNA transposons encode a transposase flanked by inverted terminal repeats that contain transposase binding sites. Any gene of interest flanked by the inverted terminal repeats can undergo transposition into the host genome via a cut and paste mechanism. SB tranposase has been shown to enable stable gene transfer into primary human T cells and its utilization for TCR gene transfer is anticipated [108].
Box 1. Potential genotoxicity of gene transfer into somatic cells.
Gene transfer into somatic cells can trigger oncogenesis as a consequence of the upregulation of cellular proto-oncogenes. Insertional mutagenesis has resulted in the development of leukemia in a subset of patients with X-linked severe combined immunodeficiency who were treated with hematopoietic stem cells that had been corrected by retrovirus-mediated gene transfer. Mature T cells have been shown to be relatively resistant to oncogene transformation as a result of retroviral insertion [104], and vector design may further reduce the genotoxic risk from vector integration and improve the safety of gene-modified T-cell therapy of malignancy. Nevertheless, this issue remains a concern for the clinical translation of therapeutics with gene-modified T cells. An additional approach to improve safety is to concurrently introduce a mechanism for conditional cell suicide, either using a drug that activates a death program or a monoclonal antibody to invoke killing by antibody-dependent cellular cytotoxicity [142–145].
The only experience using TCR gene transfer to generate T cells for adoptive therapy of human cancer has been in metastatic melanoma. In the first reported study, two out of 15 patients experienced objective tumor regressions after receiving autologous T lymphocytes engineered to express a TCR specific for the melanocyte differentiation antigen MART-1 [92]. Although this study established the therapeutic potential of genetically modified T cells for the treatment of human malignancies, issues were identified that need to be addressed to improve the results. These include safe methods for gene delivery that achieve adequate and stable TCR transgene expression, strategies for minimizing pairing of the introduced TCR chains with the endogenous TCR chains that might result in a deleterious specificity, and introducing the TCR into T cells that can establish a durable functional T-cell response in vivo after adoptive transfer.
Achieving adequate TCR expression, pairing & avidity
T-cell receptor transgene cassettes that allow the coupled transcription of both genes under control of the same promoter yielding a single coding mRNA molecule have been developed. These vectors utilize an internal ribosomal entry site or virus-derived sequences that encode self-cleaving peptides, such as 2A to promote coordinate translation of the mRNA into two separate α- and β-chain proteins. Recent studies that compared both features favored 2A elements, which allowed translation of the TCR-α and -β chains at an almost equimolar ratio and provided optimal expression of the TCR [96].
A significant concern with TCR transfer is the potential for cross-pairing of transferred with endogenous TCR chains, which could result in the formation of hybrid receptors, with potentially autoreactive specificity. Preferential pairing of the two introduced TCR-α and-β chains can be achieved by replacing the human constant regions of the TCR-α and-β chains with murine constant regions, or by incorporating cysteine residues in positions that promote disulfide bonds between the introduced chains [109]. As an alternative method, the introduction of TCR-α and -β chains into γδ T cells has been proposed to prevent TCR mispairing. However, the low frequency of γδ T cells in the peripheral blood and their preferential homing to the mucosa of the GI tract make them less attractive for adoptive immunotherapy of B-cell malignancies [110].
T-cell receptor chains specific for self-proteins are often of low affinity, and it may be necessary to improve affinity for therapeutic efficacy. This can be accomplished for a TCR of known specificity through directed in vitro evolution, enabling the engineering of TCRs with the ability to bind HLA-bound peptides at picomolar concentrations [111], or by high-throughput screening of TCR that have been mutated randomly to identify higher affinity pairs.
Chimeric antigen receptors
In 1997, the monoclonal anti-CD20-antibody rituximab was approved as the first therapeutic antibody for cancer treatment and has since revolutionized the treatment of CD20-positive B-cell tumors. The combination of rituximab with standard chemotherapy has significantly improved response and survival rates compared with chemotherapy alone [112,113]. The potential to combine the specificity of monoclonal antibodies with the advantages of a durable cellular immune response spurred the development of CARs that could be expressed in T cells. CARs typically consist of a scFv that incorporates the heavy and light variable chains (VH and VL, respectively) of a monoclonal antibody that recognizes a tumor cell molecule fused to the ζ-chain of the CD3/TCR complex to trigger T-cell activation and cytotoxicity. To improve activation after engagement of the CAR, a costimulatory signal can be provided by the addition of CD28 or other costimulatory signaling domains to the ζ-chain [114,115]. An important advantage of CAR-modified T cells is their ability to recognize tumor cells without the requirements of MHC restriction, removing obstacles, such as defects in antigen processing and low levels of MHC expression on malignant cells, that might limit the efficacy of conventional or TCR-modified T cells.
Several B-cell lineage surface molecules are retained on B-cell tumors and represent attractive targets for CAR-modified T-cell therapy. The feasibility of adoptive immunotherapy with autologous CD20-specific CAR-modified T cells has been studied in patients with NHL and mantle cell lymphoma. In a trial of seven patients, two maintained a previous complete response, one achieved a partial response and four had stable disease [116]. Modified T cells persisted for up to 5–9 weeks after infusion in some patients but at a very low level. Improving the magnitude and duration of the response achieved by adoptive transfer will be important to improve efficacy, particularly for the treatment of patients with a larger tumor burden. Other surface molecules that are expressed on B-cell tumors have been targeted with CAR-modified T cells in preclinical studies, including CD19 and CD22 [117,118], and clinical trials with CD19-redirected T cells are now in progress in several laboratories for B-ALL, B-CLL and lymphoma.
Clinical trials of T-cell therapy using CAR-modified T cells should provide insight into potential limitations of this approach. One concern is that transferred T cells might be eliminated prematurely due to a host immune response to the murine VH and VL fragments of CARs that are derived from murine antibodies. Another issue identified in preclinical work is that the stoichiometry of antibody binding to tumor cells is different from that of the TCR/MHC interaction and might not provide for optimal T-cell activation or survival in vivo. Clinical trials are required to determine how significant these issues will be. Many molecules being targeted on B-cell malignancies by CAR-modified T cells are also expressed on normal B cells, and successful therapy is likely to result in a B-cell deficiency, unless strategies are developed for the conditional elimination of transferred T cells after tumor eradication is complete.
Qualitative properties of T cells required for effective adoptive therapy
The efficacy of adoptive T-cell therapy requires that the transferred tumor-reactive T cells home to sites of tumor, mediate effector functions in the tumor environment, and persist sufficiently long in vivo to eradicate the majority or even all of the malignant cells. Preclinical models and clinical trials have shown that modifying the environment into which T cells are transferred and selecting T cells with distinct properties for adoptive transfer are important for achieving optimal anti-tumor effects.
Homing of T cells to tumor sites
The ability of adoptively transferred T cells to migrate to and penetrate large tumor masses has been shown to be an impediment to effective therapy of solid tumors in murine models [119], and may reflect alterations in homing receptors expressed on T cells, dysregulated expression of leukocyte adhesion molecules on the tumor vasculature and biophysical properties of the tumor environment that may limit T-cell infiltration [120,121]. There are limited data on the migration of human T cells administered to patients with malignancy and B-cell tumors in particular. Adoptively transferred T cells specific for EBV antigens expressed on EBV-associated post-transplant lymphoproliferative disease or HD were readily identified in tumor biopsies, suggesting that homing may be less of an obstacle for tumors that originate in lymph nodes or bone marrow, particularly if high levels of transferred T cells can be achieved and sustained in the blood [69]. Many tumors produce chemokines to attract and educate cells to have immunosuppressive functions, thus, in situations where homing of effector T cells is limited, it may be possible to improve their migration to tumor sites by introducing chemokine receptors that respond to factors produced in the tumor environment [122].
Local suppression of effector function in the tumor environment
It is increasingly apparent that the tumor environment is hostile to the development and function of effector T cells as a consequence of the local recruitment of Tregs and myeloid suppressor cells, tumor cell expression of ligands for inhibitory receptors, and/or secretion of molecules that inhibit effector T-cell function. The precise immune evasion strategies employed by B-cell malignancies are beginning to be elucidated and will be instructive for designing modifications to adoptively transferred T cells or the host to overcome immune evasion. For example, PD-L1 is present in HD and MM and promotes anergy and apoptosis of T cells after binding to programmed death-1 (PD-1) [123,124]. PD-1 expression was elevated in tumor-infiltrating and peripheral T cells of HD patients and blockade of the PD-1 signaling pathway restored IFN-γ production of tumor-infiltrating T cells, suggesting that such an approach might improve the efficacy of adoptively transferred tumor-reactive T cells [124]. Other immune escape strategies have been identified in HD and MM, including the production of TGF-β and IL-10, and strategies to render T cells resistant to these negative regulators have been described [56,125]. Finally, loss of tumor antigen expression and downregulation of HLA and costimulatory molecules, occur in B-cell malignancies, suggesting that targeting multiple epitopes might be necessary to prevent the emergence of antigen loss variants under immune selective pressure provided by adoptive T-cell transfer [126].
Recent studies by Gribben et al. have identified direct effects on gene-expression profiles of CD4+ and CD8+ T cells induced by cell-to-cell contact with B-CLL cells [127]. The affected genes are involved in cytoskeleton formation, vesicle trafficking and cytotoxicity, and the altered gene expression in T cells is accompanied by defective synapse formation with target cells, leading to impaired recognition of malignant B-CLL cells. The formation of a functional immune synapse between T cells and B-CLL could be partially restored in vitro with the immunomodulatory drug lenalidomide, suggesting its potential use to promote recognition of tumor cells by adoptively transferred T cells [128,129].
Improving persistence of adoptively transferred tumor-reactive T cells with cytokines
Studies in animal models of adoptive T-cell therapy for leukemia have demonstrated that transferred T cells must persist for long periods to mediate complete tumor rejection. Efforts to translate T-cell therapy to human malignancies have required the in vitro isolation and expansion of T cells, which may render them less fit to survive and function in vivo. One of the first strategies that improved T-cell survival after adoptive transfer was the administration of IL-2 [130]. Administration of both low-dose and high-dose IL-2 transiently improved the persistence of transferred T cells, however high-dose IL-2 was accompanied by significant side effects [131].
IL-7 and IL-15 are used less often than IL-2 for expanding T cells in vitro but have been shown to be critical for survival and proliferation of memory T cells in vivo [132,133]. IL-7 and IL-15 levels become elevated in lymphopenia and induce the proliferation of T cells to restore homeostasis [134]. It was therefore hypothesized that the induction of lymphopenia might lead to prolonged persistence of adoptively transferred T cells by reducing the competition for homeostatic cytokines, minimizing the influence of Tregs and other suppressors, and creating space in the lymphoid compartment of the recipient for the transferred T cells. The use of lymphodepleting chemotherapy alone or with irradiation prior to the transfer of melanoma-specific T cells resulted in better persistence and expansion of transferred T cells in vivo, as well as improved tumor infiltration and anti-tumor activity [135]. Further clinical studies will help to elucidate whether the administration of homeostatic cytokines alone may have a similar effect on transferred T cells and circumvent the toxicity associated with lymphodepleting chemoradiotherapy.
Selecting T cells with the intrinsic capacity to persist & function after adoptive transfer
Clinical trials of adoptive T-cell therapy for human viral diseases after allogeneic HCT using T-cell clones or polyclonal T cells derived from a healthy donor with prior exposure to the pathogen demonstrated that transferred T cells could persist long-term in vivo [64,136]. This proved not to be the case when autologous tumor-reactive T cells were isolated from cancer patients and used to treat human malignancies, and poor persistence of transferred T cells probably contributed to the lack of sustained anti-tumor efficacy [116,130,135]. An explanation for these apparently discrepant results is now emerging from work in animal models, which suggest that the distinct transcriptional programs of naive and subsets of memory T cells impart qualitative differences that are retained in effector progeny and influence cell fate after adoptive transfer (Box 2).
Box 2. Distinct programming of T cells used for adoptive therapy.
It has been suggested previously that at least some memory T cells have properties of self-renewal and differentiation, attributes typically assigned to stem cells [146]. A recent study in mice demonstrated that the initial division of a naive T cell after antigen encounter is asymmetric and endows each of the daughter cells with distinct properties that dictate their ability to function as memory cells [147]. A putative memory T stem cell has also been described in a murine model of graft-versus-host disease [148]. Taken together, this work illustrates that even similar T-cell subsets exhibit distinct programming, and has focused greater attention on how properties of T cells other than tumor specificity might influence their efficacy in adoptive immunotherapy.
Discrete phenotypic and functional subsets of T cells have been identified, including naive (TN), effector (TE), central memory (TCM) and effector memory (TEM) T cells [137]. After TCR engagement by antigen in vivo, TN cells undergo proliferation and programmed differentiation, resulting in the generation of large numbers of TE cells, most of which die as antigen is cleared, leaving a small pool of functionally distinct TCM and TEM cells that persist long-term and respond to antigen re-exposure by differentiating into a new wave of TE cells [137,138]. It has generally been assumed that TE cells isolated from TN, TCM or TEM subsets would behave similarly and have equivalent efficiency when used for adoptive T-cell therapy. Recent data in a nonhuman primate model in which antigen-specific TE cells derived from either TCM or TEM were reinfused into animals has cast doubt on this assumption. In this study, T-cell clones derived from TCM, but not those derived from TEM cells were capable of persisting long term, reacquiring the phenotype of memory cells and populating memory T-cell niches in vivo after adoptive transfer [139]. These results demonstrate that some of the progeny of clonally derived TE cells retain intrinsic programming of the parental cell of origin and provide an explanation for the inconsistent persistence of transferred T cells when unselected T cells are used as the source to generate tumor-specific T cells for adoptive immunotherapy. Moreover, these findings suggest that selecting T cells from the TCM pool for the introduction of T-cell or chimeric antigen receptors that target B-cell malignancies may ensure more uniform persistence after cell transfer. An alternative approach that has been employed is to use T cells specific for a virus, such as EBV or CMV for genetic modification, since many of these cells will be derived from TCM. This strategy also enables signaling through the endogenous TCR to potentially amplify and maintain the transferred T cells in vivo and has been used in preclinical studies with EBV-specific T cells expressing an anti-CD30ζ CAR to target HD and with some success in clinical trials with EBV-specific T cells expressing a CAR specific for neuroblastoma [140,141].
Expert commentary
The application of adoptive T-cell therapy for human malignancies, including B-cell tumors has long held attraction due to the potential for specific elimination of tumor cells without the toxicities associated with chemotherapy and radiation. There is convincing evidence from allogeneic HCT that B-cell malignancies are susceptible to elimination by T cells, however the translation of this knowledge into specific adoptive T-cell therapy has been impeded by the lack of readily available antigens to target in B-cell tumors, with the exception of Ig–Id or EBV antigens in the subset of malignancies that express EBV proteins. Efforts to identify polymorphic and nonpolymorphic tumor antigens expressed on B-cell malignancies that can be recognized by T cells have led to studies of T-cell therapy in preclinical models, and clinical trials of this approach have been initiated. The engineering of T cells to express a TCR or CAR that targets antigens and surface molecules that are broadly expressed by B-cell malignancies can overcome the obstacle of isolating tumor-reactive T cells from the endogenous repertoire, and has facilitated the initiation of clinical trials by several groups in both aggressive and indolent B-cell tumors. Critical insight has been derived into qualitative properties of T cells that ensure persistence after adoptive transfer. The evaluation of data from well-designed clinical trials and ongoing studies in animal models will determine the potential toxicity, efficacy and limitations of adoptive T-cell therapy for B-cell malignancies, and will direct subsequent studies that may lead to the establishment of adoptive T-cell transfer as a useful and broadly applicable modality.
Five-year view
The field of adoptive T-cell therapy is currently undergoing a significant resurgence, due in part to the proven efficacy of this approach in melanoma, the opportunities provided by molecular profiling of tumors to identify target antigens, and the feasibility of isolating or engineering T cells of defined specificity. Allogeneic HCT will remain a mainstay of therapy for B-cell malignancies owing to its proven curative potential, and efforts in several laboratories to identify polymorphic antigens expressed on B-cell tumors using genome-association studies will identify minor H antigens that can be targeted selectively with T-cell therapy to augment GVT reactivity without causing GVHD. If this endeavor is successful, the use of less toxic RIC regimens might be extended to younger patients with more aggressive B-cell tumors. Clinical trials of adoptive T-cell therapy for B-cell malignancies in the nontransplant setting, including those that use TCR- or CAR-modified T cells, have already been initiated and will be completed in the next 5 years. These trials will provide data on the safety and efficacy of this approach that will be used to design subsequent trials, and determine whether it is appropriate and how best to incorporate T-cell therapy into conventional therapeutic regimens. In patients who fail to respond to T-cell therapy, it will be important to analyze whether the failure of therapy reflects intrinsic properties of effector T cells, or systemic or local suppression of function in the tumor-bearing patient to provide insight into the subset of patients that can benefit most by T-cell therapy, and to derive strategies for improving outcome in patients less likely to respond.
Key issues.
Indolent B-cell malignancies are susceptible to the T-cell mediated graft-versus-tumor effect of allogeneic hematopoietic cell transplant as a consequence of recognition of polymorphic and tumor-associated antigens.
B-cell malignancies express or overexpress several nonpolymorphic proteins that have limited expression on normal tissues, and can be targeted by tumor-specific T cells present in the endogenous repertoire.
Gene-transfer techniques can be used to endow T cells with tumor specificity, either through the expression of T-cell receptor genes that recognize tumor-associated peptides displayed on MHC molecules, or through the expression of single-chain antibody fragments that are specific for B-lineage surface molecules.
Effector T cells derived from central memory precursors exhibit long-term in vivo persistence after adoptive transfer, migrate to memory T-cell niches and revert to the memory pool, suggesting that adoptive T-cell therapy for cancer employing central memory cells may have improved efficacy.
T-cell homing to tumor sites and maintenance of effector function in the immunosuppressive tumor microenvironment will be critical for the success of adoptive T-cell therapy.
Acknowledgments
This work was supported by NIH grants CA18029 (Stanley R Riddell), R01 CA114536, and the Leukemia and Lymphoma Society. Michael Hudecek is a scholar of the German Research Foundation (DFG).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Michael Hudecek, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA and Department of Hematology, Oncology and Coagulation, University of Leipzig Medical Center, University of Leipzig, Germany, Tel.: +1 206 667 4181, Fax: +1 206 667 7983, mhudecek@fhcrc.org.
Larry D Anderson, Jr, University of Texas Southwestern Medical Center, Department of Internal Medicine, Hematology–Oncology, 5323 Harry Hines Blvd, Dallas, TX 75390, USA, Tel.: +1 214 648 5906, Fax: +1 214 648 4152, larry.anderson@utsouthwestern.edu.
Tetsuya Nishida, Nagoya University Graduate School of Medicine, 65-Tsurumai-cho, Showa-ku, Nagoya, Aichi 466-8560, Japan, Tel.: +81 527 442 145, Fax: +81 527 442161, tnishida@med.nagoya-u.ac.jp.
Stanley R Riddell, Departments of Medicine, University of Washington, Seattle, WA, USA and Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, D3-100, Seattle, WA 98109, USA, Tel.: +1 206 667 5249, Fax: +1 206 667 7983, sriddell@fhcrc.org.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Maloney DG. Immunotherapy for non-Hodgkin’s lymphoma: monoclonal antibodies and vaccines. J. Clin. Oncol. 2005;23(26):6421–6428. doi: 10.1200/JCO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Kay NE, Rai KR, O’Brien S. Chronic lymphocytic leukemia: current and emerging treatment approaches. Clin. Adv. Hematol. Oncol. 2006;4(11) Suppl. 22:1–10. [PubMed] [Google Scholar]
- 3.Ravandi F, O’Brien S. Alemtuzumab in CLL and other lymphoid neoplasms. Cancer Invest. 2006;24(7):718–725. doi: 10.1080/07357900600981414. [DOI] [PubMed] [Google Scholar]
- 4.Bensinger WI. Role of autologous and allogeneic stem cell transplantation in myeloma. Leukemia. 2009;23(3):442–448. doi: 10.1038/leu.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maloney D. Allogeneic transplantation following nonmyeloablative conditioning for aggressive lymphoma. Bone Marrow Transplant. 2008;42 Suppl. 1:S35–S36. doi: 10.1038/bmt.2008.110. [DOI] [PubMed] [Google Scholar]
- 6.Rotta M, Storer BE, Sahebi F, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009;113(14):3383–3391. doi: 10.1182/blood-2008-07-170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorror ML, Storer BE, Sandpaper BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J. Clin. Oncol. 2008;26(30):4912–4920. doi: 10.1200/JCO.2007.15.4757.. • Durable complete remissions can be induced following non-myeloablative hematopoietic cell transplantation (HCT) in a subset of patients with advanced chemotherapy-refractory B-cell chronic lymphocytic leukemia (B-CLL) and provides evidence for the susceptibility of B-CLL to the T-cell-mediated graft-versus-tumor (GVT) effect. Also demonstrates that the beneficial GVT effect and detrimental graft-versus-host disease (GVHD) cannot be consistently separated with current approaches and that alternative therapeutic modalities are required to confer anti-tumor reactivity in patients who do not respond to HCT.
- 8.Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA, Schumacher TN. Immunotherapy through TCR gene transfer. Nat. Immunol. 2001;2(10):957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 9.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRξ/CD28 receptor. Nat. Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 10.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957;257(11):491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 11.Mathe G, Amiel JL, Schwarzenberg L, Cattan A, Schneider M. Adoptive immunotherapy of acute leukemia: experimental and clinical results. Cancer Res. 1965;25(9):1525–1531. [PubMed] [Google Scholar]
- 12.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 1979;300(19):1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 14.Bensinger WI, Buckner CD, Anasetti C, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88(7):2787–2793. [PubMed] [Google Scholar]
- 15.Hosing C, Saliba RM, McLaughlin P, et al. Long-term results favor allogeneic over autologous hematopoietic stem cell transplantation in patients with refractory or recurrent indolent non-Hodgkin’s lymphoma. Ann. Oncol. 2003;14(5):737–744. doi: 10.1093/annonc/mdg200. [DOI] [PubMed] [Google Scholar]
- 16.Michallet M, Corront B, Hollard D, et al. Allogeneic bone marrow transplantation in chronic lymphocytic leukemia: 17 cases. Report from the EBMTG. Bone Marrow Transplant. 1991;7(4):275–279. [PubMed] [Google Scholar]
- 17.Pavletic ZS, Arrowsmith ER, Bierman PJ, et al. Outcome of allogeneic stem cell transplantation for B cell chronic lymphocytic leukemia. Bone Marrow Transplant. 2000;25(7):717–722. doi: 10.1038/sj.bmt.1702237. [DOI] [PubMed] [Google Scholar]
- 18.Toze CL, Shepherd JD, Connors JM, et al. Allogeneic bone marrow transplantation for low-grade lymphoma and chronic lymphocytic leukemia. Bone Marrow Transplant. 2000;25(6):605–612. doi: 10.1038/sj.bmt.1702191. [DOI] [PubMed] [Google Scholar]
- 19.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 20.Bethge WA, Hegenbart U, Stuart MJ, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004;103(3):790–795. doi: 10.1182/blood-2003-07-2344. [DOI] [PubMed] [Google Scholar]
- 21.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J. Clin. Oncol. 1997;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 22.Russell NH, Byrne JL, Faulkner RD, Gilyead M, Das-Gupta EP, Haynes AP. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36(5):437–441. doi: 10.1038/sj.bmt.1705074. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury A, Gajewski JL, Liang JC, et al. Use of leukemic dendritic cells for the generation of antileukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 1997;89(4):1133–1142. [PubMed] [Google Scholar]
- 24.Chalandon Y, Passweg JR, Schmid C, et al. Outcome of patients developing GVHD after DLI given to treat CML relapse: a study by the chronic leukemia working party of the EBMT. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.177. DOI: 10.1038/bmt.2009.177 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 25.Mackinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86(4):1261–1268. [PubMed] [Google Scholar]
- 26.Simula MP, Marktel S, Fozza C, et al. Response to donor lymphocyte infusions for chronic myeloid leukemia is dose-dependent: the importance of escalating the cell dose to maximize therapeutic efficacy. Leukemia. 2007;21(5):943–948. doi: 10.1038/sj.leu.2404641. [DOI] [PubMed] [Google Scholar]
- 27.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 28.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 29.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 30.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat. Rev. Cancer. 2004;4(5):371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 31. den Haan JM, Meadows LM, Wang W, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279(5353):1054–1057. doi: 10.1126/science.279.5353.1054.. • First study to describe the molecular basis and the most common mechanism for the generation of minor histocompatibility antigens in humans – a nonsynonymous single nucleotide polymorphism that leads to the binding of peptides to HLA molecules that are recognized as ‘non-self’ by CD8+ T cells from an HLA-identical individual.
- 32.Murata M, Warren EH, Riddell SR. A human minor histocompatibility antigen resulting from differential expression due to a gene deletion. J. Exp. Med. 2003;197(10):1279–1289. doi: 10.1084/jem.20030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren EH, Vigneron NJ, Gavin MA, et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313(5792):1444–1447. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 34.Nishida T, Hudecek M, Kostic A, et al. Development of tumor-reactive T cells after nonmyeloablative allogeneic hematopoietic stem cell transplant for chronic lymphocytic leukemia. Clin. Cancer Res. 2009;15(14):4759–4768. doi: 10.1158/1078-0432.CCR-09-0199. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akatsuka Y, Nishida T, Kondo E, et al. Identification of a polymorphic gene, BCL2A1, encoding two novel hematopoietic lineage-specific minor histocompatibility antigens. J. Exp. Med. 2003;197(11):1489–1500. doi: 10.1084/jem.20021925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Rijke B, van Horssen-Zoetbrood A, Beekman JM, et al. A frameshift polymorphism in P2X5 elicits an allogeneic cytotoxic T lymphocyte response associated with remission of chronic myeloid leukemia. J. Clin. Invest. 2005;115(12):3506–3516. doi: 10.1172/JCI24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brickner AG, Evans AM, Mito JK, et al. The PANE1 gene encodes a novel human minor histocompatibility antigen that is selectively expressed in B-lymphoid cells and B-CLL. Blood. 2006;107(9):3779–3786. doi: 10.1182/blood-2005-08-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolstra H, de Rijke B, Fredrix H, et al. Bi-directional allelic recognition of the human minor histocompatibility antigen HB-1 by cytotoxic T lymphocytes. Eur. J. Immunol. 2002;32(10):2748–2758. doi: 10.1002/1521-4141(2002010)32:10<2748::AID-IMMU2748>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 39.Spaapen RM, Lokhorst HM, van den Oudenalder K, et al. Toward targeting B cell cancers with CD4+ CTLs: identification of a CD19-encoded minor histocompatibility antigen using a novel genome-wide analysis. J. Exp. Med. 2008;205(12):2863–2872. doi: 10.1084/jem.20080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spierings E, Hendriks M, Absi L, et al. Phenotype frequencies of autosomal minor histocompatibility antigens display significant differences among populations. PLoS Genet. 2007;3(6):e103. doi: 10.1371/journal.pgen.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamei M, Nannya Y, Torikai H, et al. HapMap scanning of novel human minor histocompatibility antigens. Blood. 2008;113(21):5041–5048. doi: 10.1182/blood-2008-07-171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinilla-Ibarz J, Cathcart K, Korontsvit T, et al. Vaccination of patients with chronic myelogenous leukemia with bcr–abl oncogene breakpoint fusion peptides generates specific immune responses. Blood. 2000;95(5):1781–1787. [PubMed] [Google Scholar]
- 43.Davis TA, Maloney DG, Czerwinski DK, Liles TM, Levy R. Anti-idiotype antibodies can induce long-term complete remissions in non-Hodgkin’s lymphoma without eradicating the malignant clone. Blood. 1998;92(4):1184–1190. [PubMed] [Google Scholar]
- 44.Meeker T, Lowder J, Cleary ML, et al. Emergence of idiotype variants during treatment of B-cell lymphoma with anti-idiotype antibodies. N. Engl. J. Med. 1985;312(26):1658–1665. doi: 10.1056/NEJM198506273122602. [DOI] [PubMed] [Google Scholar]
- 45.Bogen B, Malissen B, Haas W. Idiotope-specific T cell clones that recognize syngeneic immunoglobulin fragments in the context of class II molecules. Eur. J. Immunol. 1986;16(11):1373–1378. doi: 10.1002/eji.1830161110. [DOI] [PubMed] [Google Scholar]
- 46. Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N. Engl. J. Med. 1992;327(17):1209–1215. doi: 10.1056/NEJM199210223271705.. • First clinical trial of immunoglobulin idiotype vaccination showing induction of idiotype-specific humoral and cellular immune responses in patients with follicular lymphoma after active immunization with autologous idiotype protein.
- 47.Timmerman JM, Czerwinski DK, Davis TA, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99(5):1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 48.Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte–monocyte colony-stimulating factor against lymphoma. Nat. Med. 1999;5(10):1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 49.Inoges S, Rodriguez-Calvillo M, Zabalegui N, et al. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J. Natl Cancer Inst. 2006;98(18):1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- 50.Ai WZ, Tibshirani R, Taidi B, Czerwinski D, Levy R. Anti-idiotype antibody response after vaccination correlates with better overall survival in follicular lymphoma. Blood. 2009;113(23):5743–5746. doi: 10.1182/blood-2009-01-201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baskar S, Kobrin CB, Kwak LW. Autologous lymphoma vaccines induce human T cell responses against multiple, unique epitopes. J. Clin. Invest. 2004;113(10):1498–1510. doi: 10.1172/JCI20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trojan A, Schultze JL, Witzens M, et al. Immunoglobulin framework-derived peptides function as cytotoxic T-cell epitopes commonly expressed in B-cell malignancies. Nat. Med. 2000;6(6):667–672. doi: 10.1038/76243. [DOI] [PubMed] [Google Scholar]
- 53.Houot R, Levy R. Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev. 2009;23(3):137–142. doi: 10.1016/j.blre.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Osterborg A, Yi Q, Henriksson L, et al. Idiotype immunization combined with granulocyte–macrophage colony-stimulating factor in myeloma patients induced type I, major histocompatibility complex-restricted, CD8- and CD4-specific T-cell responses. Blood. 1998;91(7):2459–2466. [PubMed] [Google Scholar]
- 55.Wen YJ, Ling M, Bailey-Wood R, Lim SH. Idiotypic protein-pulsed adherent peripheral blood mononuclear cell-derived dendritic cells prime immune system in multiple myeloma. Clin. Cancer Res. 1998;4(4):957–962. [PubMed] [Google Scholar]
- 56.Brown RD, Pope B, Murray A, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7–1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-β1 and interleukin-10. Blood. 2001;98(10):2992–2998. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- 57.Bogen B, Schenck K, Munthe LA, Dembic Z. Deletion of idiotype (Id)-specific T cells in multiple myeloma. Acta Oncol. 2000;39(7):783–788. doi: 10.1080/028418600750063505. [DOI] [PubMed] [Google Scholar]
- 58.Hong S, Qian J, Yang J, Li H, Kwak LW, Yi Q. Roles of idiotype-specific T cells in myeloma cell growth and survival: Th1 and CTL cells are tumoricidal while Th2 cells promote tumor growth. Cancer Res. 2008;68(20):8456–8464. doi: 10.1158/0008-5472.CAN-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osterroth F, Garbe A, Fisch P, Veelken H. Stimulation of cytotoxic T cells against idiotype immunoglobulin of malignant lymphoma with protein-pulsed or idiotype-transduced dendritic cells. Blood. 2000;95(4):1342–1349. [PubMed] [Google Scholar]
- 60.Vera J, Savoldo B, Vigouroux S, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108(12):3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Nicola M, Zappasodi R, Carlo-Stella C, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood. 2009;113(1):18–27. doi: 10.1182/blood-2008-06-165654. [DOI] [PubMed] [Google Scholar]
- 62.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 63.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 1994;330(17):1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 64.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–1555. [PubMed] [Google Scholar]
- 65.Blake N, Lee S, Redchenko I, et al. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)- containing protein requires exogenous processing. Immunity. 1997;7(6):791–802. doi: 10.1016/s1074-7613(00)80397-0. [DOI] [PubMed] [Google Scholar]
- 66.Yin Y, Manoury B, Fahraeus R. Self-inhibition of synthesis and antigen presentation by Epstein–Barr virus-encoded EBNA1. Science. 2003;301(5638):1371–1374. doi: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 67.Bollard CM, Gottschalk S, Huls MH, et al. In vivo expansion of LMP 1- and 2-specific T-cells in a patient who received donor-derived EBV-specific T-cells after allogeneic stem cell transplantation. Leuk. Lymphoma. 2006;47(5):837–842. doi: 10.1080/10428190600604724. [DOI] [PubMed] [Google Scholar]
- 68.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110(8):2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J. Exp. Med. 2004;200(12):1623–1633. doi: 10.1084/jem.20040890.. • Demonstrates that the adoptive transfer of Epstein–Barr virus (EBV)-reactive T cells to patients with Hodgkin disease can augment CD8+ T cell immunity to the weakly immunogenic LMP-2 protein, and that the infused T cells migrate to tumor sites and promote tumor regression.
- 70.Klein U, Tu Y, Stolovitzky GA, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J. Exp. Med. 2001;194(11):1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jelinek DF, Tschumper RC, Stolovitzky GA, et al. Identification of a global gene expression signature of B-chronic lymphocytic leukemia. Mol. Cancer Res. 2003;1(5):346–361. [PubMed] [Google Scholar]
- 72.Shaughnessy J, Jr, Zhan F, Barlogie B, Stewart AK. Gene expression profiling and multiple myeloma. Best Pract. Res. Clin. Haematol. 2005;18(4):537–552. doi: 10.1016/j.beha.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Mayr C, Bund D, Schlee M, et al. MDM2 is recognized as a tumor-associated antigen in chronic lymphocytic leukemia by CD8+ autologous T lymphocytes. Exp. Hematol. 2006;34(1):44–53. doi: 10.1016/j.exphem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 74.Mayr C, Bund D, Schlee M, et al. Fibromodulin as a novel tumor-associated antigen (TAA) in chronic lymphocytic leukemia (CLL), which allows expansion of specific CD8+ autologous T lymphocytes. Blood. 2005;105(4):1566–1573. doi: 10.1182/blood-2004-04-1233. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt SM, Schag K, Muller MR, et al. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102(2):571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 76.Frank C, Hundemer M, Ho AD, Goldschmidt H, Witzens-Harig M. Cellular immune responses against the cancer-testis antigen SPAN-XB in healthy donors and patients with multiple myeloma. Leuk. Lymphoma. 2008;49(4):779–785. doi: 10.1080/10428190801911688. [DOI] [PubMed] [Google Scholar]
- 77.Hundemer M, Schmidt S, Condomines M, et al. Identification of a new HLA-A2- restricted T-cell epitope within HM1.24 as immunotherapy target for multiple myeloma. Exp. Hematol. 2006;34(4):486–496. doi: 10.1016/j.exphem.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110(5):1587–1594. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatta Y, Takeuchi J, Saitoh T, et al. WT1 expression level and clinical factors in multiple myeloma. J. Exp. Clin. Cancer Res. 2005;24(4):595–599. [PubMed] [Google Scholar]
- 80.van Baren N, Brasseur F, Godelaine D, et al. Genes encoding tumor-specific antigens are expressed in human myeloma cells. Blood. 1999;94(4):1156–1164. [PubMed] [Google Scholar]
- 81.Menssen HD, Renkl HJ, Rodeck U, et al. Presence of Wilms’ tumor gene (wt1) transcripts and the WT1 nuclear protein in the majority of human acute leukemias. Leukemia. 1995;9(6):1060–1067. [PubMed] [Google Scholar]
- 82.Doubrovina ES, Doubrovin MM, Lee S, et al. In vitro stimulation with WT1 peptide-loaded Epstein–Barr virus-positive B cells elicits high frequencies of WT1 peptide-specific T cells with in vitro and in vivo tumoricidal activity. Clin. Cancer Res. 2004;10(21):7207–7219. doi: 10.1158/1078-0432.CCR-04-1040. [DOI] [PubMed] [Google Scholar]
- 83.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J. Immunol. Methods. 2006;310(1–2):40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 84.Rezvani K, Yong AS, Savani BN, et al. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia. Blood. 2007;110(6):1924–1932. doi: 10.1182/blood-2007-03-076844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsuboi A, Oka Y, Nakajima H, et al. Wilms tumor gene WT1 peptide-based immunotherapy induced a minimal response in a patient with advanced therapy-resistant multiple myeloma. Int. J. Hematol. 2007;86(5):414–417. doi: 10.1007/BF02983998. [DOI] [PubMed] [Google Scholar]
- 86.Goodyear O, Piper K, Khan N, et al. CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood. 2005;106(13):4217–4224. doi: 10.1182/blood-2005-02-0563. [DOI] [PubMed] [Google Scholar]
- 87.van Rhee F, Szmania SM, Zhan F, et al. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood. 2005;105(10):3939–3944. doi: 10.1182/blood-2004-09-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251.. •• Describes the regression of melanoma metastases following adoptive transfer of an autologous CD4+ T-cell clone specific for the NY-ESO-1 antigen and underlines the potential use of CD4+ T cells in adoptive T-cell therapy for human malignancies.
- 89.Atanackovic D, Luetkens T, Hildebrandt Y, et al. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin. Cancer Res. 2009;15(4):1343–1352. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- 90.Dembic Z, Haas W, Weiss S, et al. Transfer of specificity by murine α and β T-cell receptor genes. Nature. 1986;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 91.Mommaas B, van Halteren AG, Pool J, et al. Adult and cord blood T cells can acquire HA-1 specificity through HA-1 T-cell receptor gene transfer. Haematologica. 2005;90(10):1415–1421. [PubMed] [Google Scholar]
- 92. Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003.. •• First study using genetically engineered autologous T cells to express a tumor-reactive T cell receptor (TCR) by retrovirus-mediated gene transfer. The study showed tumor regression in a small fraction of patients with metastatic melanoma and revealed several challenges associated with this approach including the requirement for high levels of persistent TCR transgene expression.
- 93.Stanislawski T, Voss RH, Lotz C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2001;2(10):962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J. Immunol. 2005;174(7):4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Engels B, Cam H, Schuler T, et al. Retroviral vectors for high-level transgene expression in T lymphocytes. Hum. Gene Ther. 2003;14(12):1155–1168. doi: 10.1089/104303403322167993. [DOI] [PubMed] [Google Scholar]
- 96.Yang S, Cohen CJ, Peng PD, et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15(21):1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Costello E, Munoz M, Buetti E, Meylan PR, Diggelmann H, Thali M. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7(7):596–604. doi: 10.1038/sj.gt.3301135. [DOI] [PubMed] [Google Scholar]
- 98.Cavalieri S, Cazzaniga S, Geuna M, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102(2):497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 99.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300(5626):1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 100.Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 101. Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118(9):3143–3150. doi: 10.1172/JCI35798.. • Reports the activation of an oncogene as a consequence of γ-retroviral vector integration and occurrence of clonal T-cell proliferation in a clinical gene therapy trial for X-linked SCID.
- 102.Thornhill SI, Schambach A, Howe SJ, et al. Self-inactivating gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency. Mol. Ther. 2008;16(3):590–598. doi: 10.1038/sj.mt.6300393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Montini E, Cesana D, Schmidt M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119(4):964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Newrzela S, Cornils K, Li Z, et al. Resistance of mature T cells to oncogene transformation. Blood. 2008;112(6):2278–2286. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- 105.Jensen MC, Clarke P, Tan G, et al. Human T lymphocyte genetic modification with naked DNA. Mol. Ther. 2000;1(1):49–55. doi: 10.1006/mthe.1999.0012. [DOI] [PubMed] [Google Scholar]
- 106.Schaft N, Dorrie J, Muller I, et al. A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer Immunol. Immunother. 2006;55(9):1132–1141. doi: 10.1007/s00262-005-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ivics Z, Hackett PB, Plasterk RH, Izsvak Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91(4):501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 108.Huang X, Wilber AC, Bao L, et al. Stable gene transfer and expression in human primary T cells by the Sleeping Beauty transposon system. Blood. 2006;107(2):483–491. doi: 10.1182/blood-2005-05-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuball J, Dossett ML, Wolfl M, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109(6):2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Veken LT, Coccoris M, Swart E, Falkenburg JH, Schumacher TN, Heemskerk MH. αβ T cell receptor transfer to γ δ T cells generates functional effector cells without mixed TCR dimers in vivo. J. Immunol. 2009;182(1):164–170. doi: 10.4049/jimmunol.182.1.164. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Moysey R, Molloy PE, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat. Biotechnol. 2005;23(3):349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 112.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 113.Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2005;23(18):4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 114.Schumacher TN. T-cell-receptor gene therapy. Nat. Rev. Immunol. 2002;2(7):512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 115.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 116.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 118.James SE, Greenberg PD, Jensen MC, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 2008;180(10):7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ochsenbein AF, Sierro S, Odermatt B, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411(6841):1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 120.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 121.Weishaupt C, Munoz KN, Buzney E, Kupper TS, Fuhlbrigge RC. T-cell distribution and adhesion receptor expression in metastatic melanoma. Clin. Cancer Res. 2007;13(9):2549–2556. doi: 10.1158/1078-0432.CCR-06-2450. [DOI] [PubMed] [Google Scholar]
- 122.Kershaw MH, Wang G, Westwood JA, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum. Gene Ther. 2002;13(16):1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 123.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD–1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 125.Foster AE, Dotti G, Lu A, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-β receptor. J. Immunother. 2008;31(5):500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rimsza LM, Roberts RA, Miller TP, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood. 2004;103(11):4251–4258. doi: 10.1182/blood-2003-07-2365. [DOI] [PubMed] [Google Scholar]
- 127.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J. Clin. Invest. 2005;115(7):1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J. Clin. Invest. 2008;118(7):2427–2437. doi: 10.1172/JCI35017.. •• Demonstrates that B-CLL cells form deficient immune synapses with autologous and allogeneic T cells from tumor-bearing patients to escape immune recognition. The formation of functional immune synapses could be restored after treatment with the immunmodulatory drug lenalidomide. These findings illustrate the need to overcome mechanisms employed by malignant cells that lead to suppression of T-cell effector function in the tumor environment.
- 129.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigenspecific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37(2–3):117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 132.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 133.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168(10):4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 134.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195(12):1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449.. •• Autologous tumor-infiltrating lymphocytes from patients with metastatic melanoma were expanded in vitro and reinfused into patients after administration of lymphodepleting preparative regimens. Host lymphodepletion was associated with increased serum levels of the homeostatic lymphocyte cytokines IL-7 and IL-15 and the objective response rate was superior to historic studies where tumor-infiltrating lymphocytes were administered without lymphodepleting chemotherapy.
- 136.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 137.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 138.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 2005;17(3):326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 139. Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103.. •• Tracked the fate of clonally derived effector T cells isolated from either central memory and effector memory precursors in a nonhuman primate model. Effector T cells derived from central memory precursors persisted long-term after adoptive transfer, required memory phenotype and reverted to both central and effector memory cells whereas effector memory-derived T-cell clones rapidly underwent apoptosis. These findings suggest that deriving tumor antigen-specific T cells from central memory precursors will provide a cell population with superior intrinsic capacity to persist after adoptive transfer.
- 140.Savoldo B, Rooney CM, Di Stasi A, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30ξ artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110(7):2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Berger C, Blau CA, Huang ML, et al. Pharmacologically regulated Fas-mediated death of adoptively transferred T cells in a nonhuman primate model. Blood. 2004;103(4):1261–1269. doi: 10.1182/blood-2003-08-2908. [DOI] [PubMed] [Google Scholar]
- 143.Kieback E, Charo J, Sommermeyer D, Blankenstein T, Uckert W. A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proc. Natl Acad. Sci. USA. 2008;105(2):623–628. doi: 10.1073/pnas.0710198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–445. [PubMed] [Google Scholar]
- 145.Tiberghien P, Reynolds CW, Keller J, et al. Ganciclovir treatment of herpes simplex thymidine kinase-transduced primary T lymphocytes: an approach for specific in vivo donor T-cell depletion after bone marrow transplantation? Blood. 1994;84(4):1333–1341. [PubMed] [Google Scholar]
- 146.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293(5528):248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 147.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315(5819):1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat. Med. 2005;11(12):1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 149.Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10(3):456–459. [PubMed] [Google Scholar]
- 150.Giannopoulos K, Li L, Bojarska-Junak A, et al. Expression of RHAMM/CD168 and other tumor-associated antigens in patients with B-cell chronic lymphocytic leukemia. Int. J. Oncol. 2006;29(1):95–103. [PubMed] [Google Scholar]