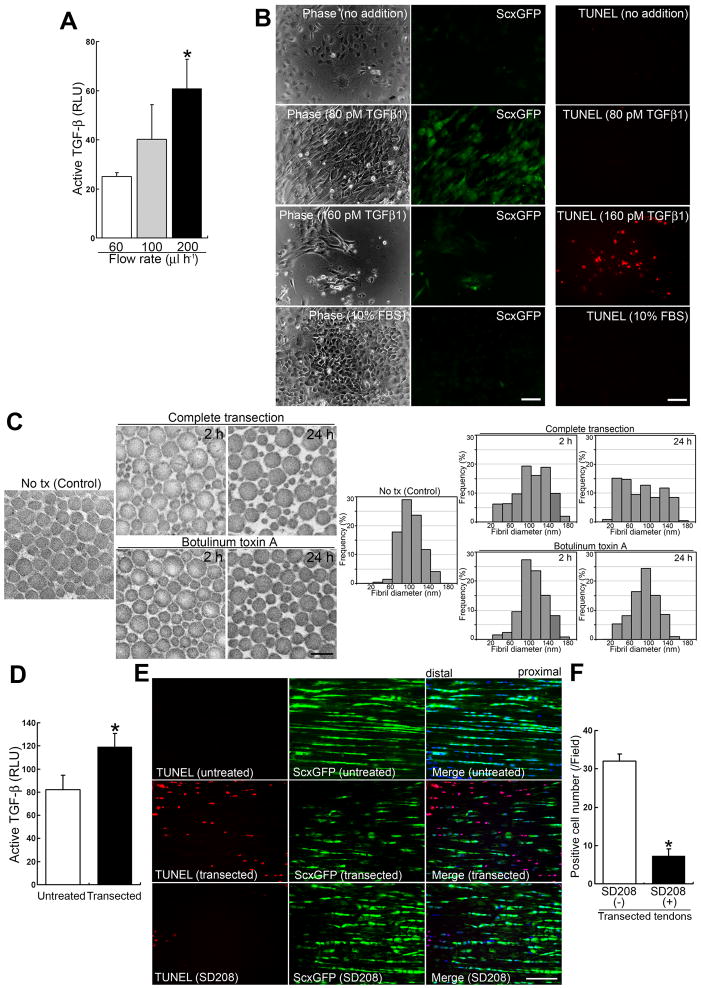
Figure 4. Sudden interruption of continuous tensile loading destabilizes the ECM’s structural organization and allows consequent release of a significant amount of active TGF-β.
(A) Active TGF-β bioassay in culture media from adult tenocyte cell lines cultured in a single network chamber for 5 d under different mechanical forces. Flow rate conditions for 60, 100 and 200 μl h−1 corresponded to 0.01, 0.015 and 0.03 dyne cm−2 mechanical force, respectively. Culture medium (100 μl) from each condition was used for the assay. The data show the amounts released in conditioned media for 1 h under different flow rates. Luciferase activity is presented as relative light units (RLU). Error bars represent standard deviation (n = 3). The values for active TGF-β released in the conditioned media over 1 h were 0.30 ± 0.018 pM under 60 μl h−1 shear stress; 0.52 ± 0.18 pM under 100 μl h−1 shear stress; and 1.01 ± 0.19 pM under 200 μl h−1 shear stress. *, significantly different (P < 0.01) compared to the amount under strain at 60 μl h−1 flow rate.
(B) Effects of the cytokine TGF-β1 on primary tenocytes. Left and middle panels: Phase contrast and fluorescence micrographs of the same area with a GFP filter for each condition. Right panels: The assessment of cell death by TUNEL staining (red). Primary tenocytes were cultured for 48 h, then cytokines were added for a further 7 d. Note that the addition of TGF-β1 at a low concentration (2 ng ml−1; 80 pM) retains the expression of ScxGFP. In contrast to other culture conditions, only a higher concentration (160 pM) leads to tenocyte cell death (13.0 ± 2.7 cells/field [n = 4; field = 0.15 mm2]). Retention of ScxGFP expression was seen at TGF-β1 concentrations as low as 20 pM (data not shown). Neither no addition (DMEM containing 1% FBS) nor 10% FBS maintained ScxGFP expression. Bars = 100 μm.
(C) Ultrastructural analysis of collagen fibrils. Transmission electron micrographs of transverse sections (left panels) and morphometric analysis of fibrils (right panels) in intact (Control), completely transected, and toxin-injected adult Achilles tendon tissues at 2 and 24 h post treatment. Note that collagen fibril ultrastructure in tension-collapsing tendons by complete transection reveals irregular patterns by as early as 2 h post injury. Bar in left panel = 200 nm.
(D) Active TGF-β bioassay in intact (untreated) and transected (at 1.5 h after complete transection) adult Achilles tendons. Luciferase activity is presented as relative light units (RLU). Error bars represent standard deviation (n = 3). Note that the transacted tendon tissues release significant amounts of active TGF-β. *, P < 0.05.
(E) Effects of SD208 on cell death in vivo after complete transection of adult Achilles tendon. TUNEL staining (red; left panels), expression of ScxGFP (green; middle panels) and merged images (right panels) with DAPI (blue) in intact tendons and at 1.5 h after complete transection without (untreated) or with 1.0 μM SD208 treatment. Bar = 50 μm.
(F) Analysis of TUNEL-positive cells at 1.5 h after complete transection without or with SD208 treatment. Error bars represent standard deviation (n = 4; field = 0.07 mm2). Note that SD208 significantly attenuates tenocyte cell death. *, P < 0.001.