Abstract
High conductance calcium-activated potassium (BKCa) channels can modulate cell excitability and neurotransmitter release at synaptic and afferent terminals. BKCa channels are present in primary afferents of most, if not, all internal organs and are an intriguing target for pharmacological manipulation of visceral sensation. Our laboratory has a long-standing interest in the neurophysiological differences between myelinated and unmyelinated visceral afferent function. Here, we seek to determine whether there is a differential distribution of BKCa channels in myelinated and unmyelinated vagal afferents. Immunocytochemistry studies with double staining for the BK-type KCa1.1 channel protein and isolectin B4 (IB4), a reliable marker of unmyelinated peripheral afferents, reveal a pattern of IB4 labeling that strongly correlates with the expression of the KCa1.1 channel protein. Measures of cell size and immunostaining intensity for KCa1.1 and IB4 cluster into two statistically distinct (P < 0.05) populations of cells. Smaller diameter neurons most often presented with strong IB4 labeling and are presumed to be unmyelinated (n = 1,390) vagal afferents. Larger diameter neurons most often lacked or exhibited a very weak IB4 labeling and are presumed to be myelinated (n = 58) vagal afferents. Complimentary electrophysiological studies reveal that the BKCa channel blockers charybdotoxin (ChTX) and iberiotoxin (IbTX) bring about a comparable elevation in excitability and action potential widening in unmyelinated neurons but had no effect on the excitability of myelinated vagal afferents. This study is the first to demonstrate using combined immunohistochemical and electrophysiological techniques that KCa1.1 channels are uniquely expressed in unmyelinated C-type vagal afferents and do not contribute to the dynamic discharge characteristics of myelinated A-type vagal afferents. This unique functional distribution of BK-type KCa channels may provide an opportunity for afferent selective pharmacological intervention across a wide range of visceral pathophysiologies, particularly those with a reflexogenic etiology and pain.
Keywords: sensory neurons, visceral function, charybdotoxin, iberiotoxin, A- and C-type vagal afferents
immunohistochemical and cellular electrophysiological studies have demonstrated that large conductance (BK-type) Ca+2-activated K+ (KCa) channels are present in primary vagal afferent neurons (8, 14, 22, 30). Beyond participating in membrane repolarization and contributing to the trajectory of the after hyperpolarization, the integrative physiological function associated with this subtype of KCa channel remains poorly understood. Recently, it has been shown that prostaglandin-associated excitability of aortic baroreceptor and vagal neurons of unspecified sensory modality may be mediated by inhibition of charybdotoxin (ChTX)-sensitive BK-type channels (30, 43). Furthermore, novel therapeutic agents that selectively target ChTX-sensitive BK-type channels have shown potential for the treatment of bowel disorders that intensify visceral nociception (42). There is considerable support in the literature that ChTX-sensitive BK-type KCa channels may represent a common pathway for modulation of sensory nerves in the airways with potential as a selective target for inhibition of the cough reflex (2, 44). While comparable physiological roles for the small (SK-type) and intermediate (IK-type) conductance KCa channels have been demonstrated in dorsal root ganglia neurons and in particular somatic nociceptive afferents, multiple studies have failed to implicate such a direct role for these two smaller conductance KCa channel currents in vagal afferents (1, 5, 24, 32, 44, 47).
Collectively, these studies have place added emphasis on the need for elucidating the particular role(s) of BK-type KCa channels relative to the integrative physiological function of vagal afferent pathways. In that regard, using an intact rat vagal ganglion preparation, we previously demonstrated that frequency-dependent action potential broadening occurs in vagal neurons with unmyelinated but not myelinated afferent fibers (26). BK-type KCa channels have been shown to be associated with action potential broadening and augmented firing properties in somatic afferents of the dorsal root ganglia, but the extent to which BK-type KCa channels may function similarly in vagal afferents is unknown (29, 41). In this study, we investigated the possibility that a differential distribution in the functional expression of BK-type KCa channels may represent a potential ionic mechanism underlying the contrasting discharge characteristics of myelinated and unmyelinated vagal afferents. This is important because the selective activation of unmyelinated or myelinated visceral afferents, be it by electrical, pharmacological, or physiological stimuli, often elicits vastly different reflexogenic responses from the autonomic nervous system (ANS) (4, 15, 31). It has long been recognized that dysfunction of the ANS, either as a root cause of particular pathophysiologies or by way of reflex pathways leading to the exacerbation of clinical symptoms arising from organ system disease, is inextricably linked to vagal afferent physiology (4, 34, 46, 47). Advances in pharmacological interventions as well as a more integrative understanding of afferent-mediated ANS dysfunction would benefit greatly from a more comprehensive understanding of the relative distribution of the ionic mechanisms that serve to differentiate the neurophysiological properties between unmyelinated and myelinated vagal afferents (6, 38, 48).
Here, using immunohistochemical techniques, we present evidence for a preferential expression of the KCa1.1 BK-type channel subtype in unmyelinated vagal afferent neurons of male rat. The functional implications for such an expression pattern are further investigated using an intact ganglion preparation for patch-clamp electrophysiological study of action potential dynamics from vagal neurons of known afferent conduction velocity (CV). Application of the BK-type channel antagonists ChTX and iberiotoxin similarly broadens the somatic action potential and increase the excitability of unmyelinated vagal afferents, while having no measurable electrophysiological effects on myelinated afferents. We further document that this differential expression extends from neonatal (P5–9) through maturation to adult rat (>250 g) albeit with subtle functional differences suggestive of ontogeny in the voltage- and/or Ca+2-dependent properties of the BK-type KCa channel. In addition to demonstrating a fiber-specific distribution of BK-type channels, these experimental observations provide further clarity concerning the differential ionic mechanism for integration of sensory information arising from unmyelinated and myelinated vagal afferents in rat.
MATERIALS AND METHODS
Sprague-Dawley (Harlan, Indianapolis) rat pups (5- to 9-day-old) of either gender or adult males (>250 g) were used in all electrophysiological studies. Young adult male (P49) Sprague-Dawley rats were used for immunocytochemistry. All protocols were approved by the Institutional Animal Care and Use Committees of the Purdue School of Science; Indiana University Purdue University, Indianapolis; or Case Western Reserve University.
Immunocytochemistry.
Left side vagal ganglia were isolated, quick frozen, and cryosectioned. Horizontal serial sections of 8-μm thickness were collected onto glass slides and fixed with cold 4% paraformaldehyde for 30 min. Sections were blocked for nonspecific staining in PBS containing 10% normal donkey serum, 0.3% Triton-X 100 (Pierce), and 1% BSA (Jackson Immunoresearch Laboratories) followed by overnight incubation in a primary antibody cocktail consisting of anti-BKCa (1:50; mouse; NeuroMab) or anti-BKCa (1:200; rabbit; Alomone) in PBS containing 0.3% Triton-X 100 and 1% BSA. Secondary antibodies (1:400) from appropriate hosts (Jackson Laboratories) labeled with rhodamine red-X were diluted in PBS containing 0.3% Triton X-100 and 10% donkey serum for 90 min at room temperature. Before imaging the slides were incubated with Lectin from Bandeiraea simplicifolia (Griffonia simplicifolia) conjugated to FITC (IB4; Sigma) 1:200 for 10 min. The slides were washed briefly in PBS and coverslipped using Vectashield mounting medium with DAPI. Imaging was carried out using a Nikon E600 microscope with a SPOT RT digital camera and its associated software (Diagnostic Instruments).
The resultant 8-bit images were imported into Metamorph (Version 6.1, Molecular Devices). The flatten background command was utilized to mitigate false variations in brightness. Only those neurons with a DAPI-labeled nucleus were selected and manually outlined for analysis. Cell area and intensity of anti-BKCa and IB4 fluorescence were measured using Metamorph software. The raw measures of cell area were normalized to the maximum neuronal area measured across each tissue slice. The fluorescence intensity of anti-BKCa and IB4 for each neuron was normalized to the maximum intensity measured also within each tissue slice. Restricting data normalization to measures collected from an individual tissue slice was done to reduce bias that may arise from uncontrollable factors such as orientation of the tissue slice through a particular ganglia, depth of the tissue slice into the ganglia, depth of the slice through a cell body, cell packing within the ganglia, etc. This database of normalized measures was interrogated using an automated cluster analysis algorithm (MATLAB, The MathWorks) to determine if the joint distribution of these measures could be segregated into two statistically distinct subpopulations. The difference between the identified subgroups was further tested by analysis of variance and Tukey's tests (α = 0.05).
Preparation of intact ganglia for patch recording of vagal neurons.
Slices of vagal ganglia with intact axons were prepared in a manner previously described (27). Briefly, anesthetized animals were guillotined at the midauxiliary region, preserving a length of vagus nerve suitable for measure of fiber CV. The entire vagal ganglion with attached nerve trunk was excised under stereomicroscopy (×40), and the tissue immediately was placed in chilled (4°C) recording solution (see below). The interior of the ganglion was exposed by taking a small slice from the surface of the capsule, and the ganglion and attached nerve were placed in a solution of Earle's balance salt solution (Sigma, St. Louis, MO) containing type II vollagenase (1.0 mg/ml) for 40–45 min at 37°C followed by a solution containing Trypsin-3X (5 mg/ml) for an additional 20–22 min at 37°C. The ganglion and attached nerve were moved to the recording chamber and perfused with room temperature recording solution for at least 1 h before study.
Preparation of isolated vagal ganglion neurons for patch recording.
All procedures for dissection and enzymatic isolation of vagal ganglion neurons (VGN) were identical to those previously reported (40). Briefly, for each day of experiments the entire vagal ganglia from at least two rat pups were excised bilaterally and placed in a chilled (4–8°C) vagal complete media (VCM) consisting of DMEM F-12 (Invitrogen), 5% FBS (HyClone), 0.01% penicillin-streptomycin (Sigma), and 0.1% MITO + serum extender (Becton Dickinson). The whole ganglia were digested using Trypsin-3X (5 mg/ml; Worthington) for 30 min at 37°C. The enzyme solution was replaced with VCM, and the ganglia were titrated with an aspiration pipette. The cell bodies were plated on poly-d-lysine (Sigma) coated coverslips for 3–6 h at 37°C in a high humidity, 97% room air, and 3% CO2 environment before patch recording.
Recording solutions.
For all current-clamp recordings of action potential discharge, the extracellular solution consisted of the following (in mM): 137 NaCl, 5.4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES with pH adjusted to 7.35 using 1 N NaOH. For all voltage-clamp recordings of K+ currents, the extracellular solution consisted of the following (in mM): 137 NMDG, 5.4 KCl, 1 MgCl2, 10 glucose, 10 HEPES, and nominally Ca+2 free with pH adjusted to 7.3 using 1 N HCl. The pipette solution for both recording protocols contained the following (in mM): 140 potassium aspartate, 3 MgCl2, 4 BAPTA-K, 10 HEPES, and 0.25 CaCl2 for a final buffered intracellular calcium concentration of 10 nM with pH adjusted to 7.3 using 1.3 ml of 1 N KOH per 100-ml stock for a final intacellular potassium ion concentration of ∼153 mM. Just before recording, 2 mM Mg-ATP was added to the pipette solution from a stock solution. Osmolarities of all extracellular and pipette solutions were adjusted using d-manitol (Sigma) to 310 and 290, respectively. A stock of 100 nM ChTX (Alomone) or 100 nM of iberiotoxin (IbTX; Alomone) was prepared fresh for each day of experiments and applied using a perfusion pipette or bath perfused depending on whether the patched VGN was from an isolated or intact ganglion preparation, respectively. All recordings were carried out at room temperatures (20–23°C).
Electrophysiological techniques.
The whole cell patch technique was used to carry out the voltage and current-clamp recording protocols using an Axoclamp 700A. Borosilicate glass pipettes (Sutter) were pulled and polished down to a resistance of 1–2 MΩ. Following correction for all offsets, a giga-ohm seal was formed and the pipette capacitance was compensated. Upon going whole cell the total cell capacitance (30–50 pF) and electrode access resistance (3–5 MΩ) were also compensated (70–80%). For current-clamp recordings using an intact ganglion, a Pt-IR bipolar stimulation electrode was positioned a measured distance from the recording electrode. A 500-μs monophasic current pulse was used to depolarize the afferent fiber arising from the VGN under study. The time between the stimulus artifact and arrival of the propagating action potential at the patch electrode was used in calculating fiber CV. A VGN was classified as a myelinated (A-type) or unmyelinated (C-type) afferent if fiber CV was >10 m/s or <2 m/s, respectively (27). Single somatic action potentials were also elicited using a 500-μs current pulse delivered through the patch electrode while step depolarizing currents were used to evoke repetitive discharge. For isolated VGN, we have previously demonstrated a methodology whereby select measures of action potential wave shape can be reliably used to classify an isolated neuron as either a myelinated A-type or unmyelinated C-type afferent (28). All voltage-clamp recordings were preceded by one of these current-clamp protocols for afferent classification before exchange of the bathing solution for one suitable for recording whole cell K+ currents. The voltage-clamp protocols consisted of 300-ms steps delivered at 3-s intervals from a holding potential of −80 mV to +40 mV in increments of 10 mV. Recordings were low pass filtered to 10 KHz and digitized at 50 KHz. The experimental protocols, data collection, and preliminary analysis were carried out using pCLAMP 9 and the Digidata 1322A (Axon Instruments). Corrections for liquid junction potentials were taken into consideration before final analysis of the data. Averaged measures were tabulated as means ± SD. A two-sided paired Student's t-test was used to assess statistical significance (P < 0.05) of the impact of the channel antagonists.
RESULTS
The immunohistochemical and electrophysiological studies were carried out independently in the Kunze and Schild laboratories, respectively.
Immunohistochemical staining of KCa1.1 in vagal neurons.
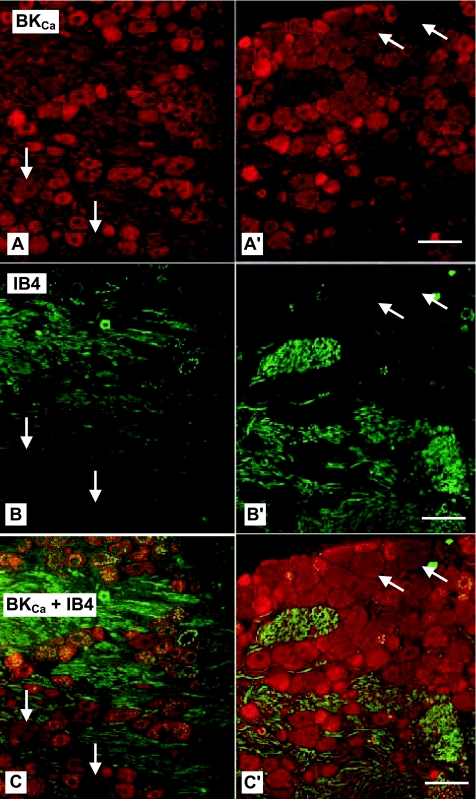
The immunohistochemical studies made use of 26, 8-μm thick horizontal sections. Fluorescence illumination of the BK-type KCa (KCNMA1) antibody shows diffuse labeling of all neurons throughout the ganglia, albeit with varying levels of intensity from section to section (Fig. 1, A and A′, arrows). No obvious differences in labeling intensity are noted between the mouse (NeuroMab) and rabbit (Alomone) antibodies, and therefore the two results are combined for purposes of analysis. Those VGN with the largest cross-sectional diameters exhibit the lightest staining for not only the KCa1.1 channel but also IB4, a reliable marker for sensory neurons with unmyelinated fibers (Fig. 1, B and B′, arrows). Superposition of these images clearly demonstrates a lack of dual labeling in these larger, presumably myelinated VGN (arrows). In stark contrast, nearly all of the smaller diameter VGN are prominently labeled for both the BK-type KCa1.1 antibody (red) and IB4 lectin (green). Superposition of these images shows dual labeling in the smaller, presumably unmyelinated VGN (Fig. 1, C and C′, yellow). These images provide strong, albeit qualitative, evidence that KCa1.1 is more prominently expressed in unmyelinated than myelinated VGN. However, the subjective nature of such visual inspection and the inherent variability in the fluorescence emissions between sections (Fig. 1, left and right, presents two extreme examples) necessitate a more rigorous quantification of KCa1.1 distribution relative to afferent fiber type.
Fig. 1.
Fluorescent images of vagal ganglion neurons (VGN) immunostained for high conductance calcium-activated potassium (BKCa) channels and isolectin B4 (IB4). Horizontal sections (8 μm) from 2 left vagal ganglia of 49-day-old male rats incubated with a cocktail containing antibodies against BKCa (A and A′; red) and IB4 (B and B′; green). C and C′: superposition of BKCa (A) and IB4 (B) fluorescent images reveal as yellow those cells containing both the BKCa and IB4 protein. Four large neurons (arrows) with little IB4 and BKCa immunoreactivity are surrounded by cells strongly labeled for BKCa (red), IB4 (green), or both (yellow). Scale bar = 50 μm for all images.
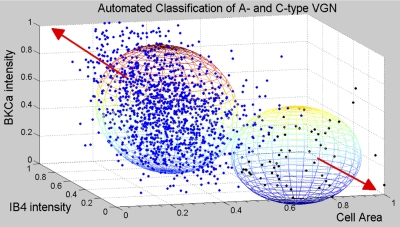
Measures of circumscribed cell area and average pixel intensity associated with the immunostaining for the KCa1.1 channel protein (Fig. 1, A and A′, red) and IB4 (Fig. 1, B and B′, green) are carried out on each neuron (n = 1,448) with clear nuclear staining (DAPI). Measures from each tissue slice are normalized for each tissue slice and sorted into a hierarchical cluster tree. The clustering algorithm reveals two subpopulations of neurons with joint distributions of cell area (or equivalent diameter) and intensity of KCa1.1 and IB4 immunostaining that are statistically distinct (Fig. 2, spheres demarcate 2 SD from the population mean, i.e., P < 0.05). The larger subgroup (n = 1,390) has an average cell area of 615.4 ± 216 μm2 (27.5 ± 5 μm diameter) and normalized IB4 and KCa1.1 immunostaining intensities of 0.53 ± 0.2 and 0.51 ± 0.2, respectively. The smaller subgroup has an average cell area of 1,315.9 ± 211 μm2 (40.8 ± 3 μm diameter) and normalized IB4 and KCa1.1 immunostaining intensities of 0.18 ± 0.1 and 0.25 ± 0.2, respectively. A two-tailed Student's t-test shows that the measurement means for these two subgroups are significantly different (P < 0.01). Additional analysis using an ANOVA and Tukey's test confirms that all but two combinations of measurement means are significantly different at the 0.05 level. The two combinations that fail the Tukey's test are the normalized IB4 and KCa1.1 immunostaining intensities for each individual subgroup, which can reasonably be expected should these two markers similarly vary in intensity according to neuronal cell type.
Fig. 2.
Classification of cell type according to BKCa and IB4 immunostaining intensity. Measures of cell area and the fluorescence intensity of the BKCa and IB4 immunostaining from 24 tissue sections (Fig. 1) from a total of 4 ganglia yielded a database consisting of 1,448 separate neurons. An automated cluster analysis (see materials and methods) segregated these normalized data into 2 distinct populations presumably representing unmyelinated C-type (n = 1,390, blue dots) and myelinated A-type (n = 58, black dots) VGN. Spheroids centered at the intersection of the population means for each of the 3 measures are drawn with a radii equal to 2 SD. Lack of overlap is evidence that the 2 populations are distinct and statistically unique (P < 0.05). Population data and additional statistical analyses are also presented in results. Red arrows indicate trending of the data toward an ideal A-type VGN, one with larger diameter but low intensity IB4 and BKCa fluorescence, or an ideal C-type VGN, one with smaller diameter and high intensity IB4 and BKCa fluorescence.
Functional impact of BK-type KCa channel antagonists on vagal afferents of known fiber type.
Following the formation of a successful patch the intrinsic resting membrane potential (RMP) is measured for each VGN. This intrinsic RMP is then adjusted to −60 mV using a small magnitude (5–10 pA) depolarizing background current. A uniform RMP increases the likelihood that from cell to cell all subtypes of voltage-dependent ion channels present in the VGN are operating from a consistent gating probability (39). A common objective for both the current-clamp and voltage-clamp recordings is to determine if the BK-type KCa channel antagonists have similar effects on the action potential discharge characteristics and whole cell K+ current of myelinated and unmyelinated VGN.
Differential effects of ChTX on action potential discharge of A- and C-type VGN from adult male rat.
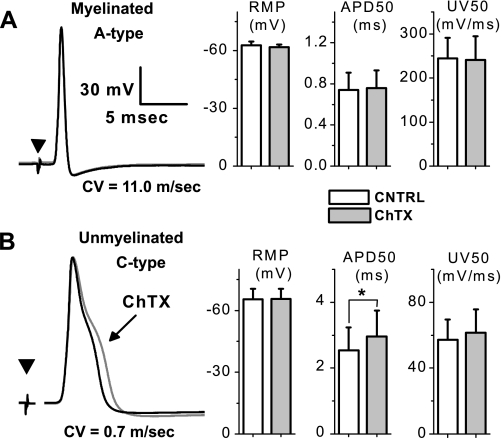
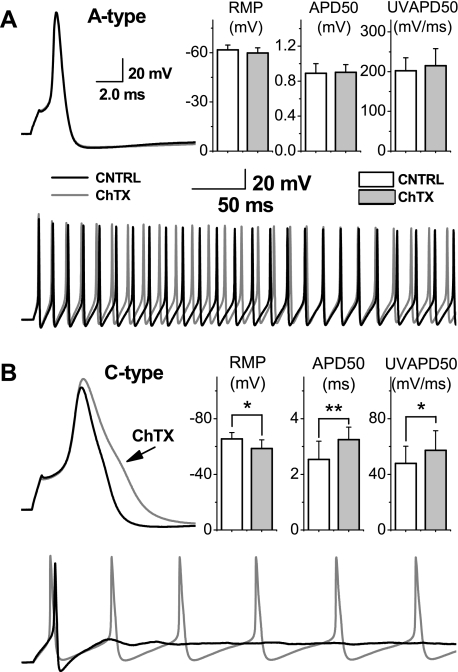
Electrical stimulation of the vagus nerve elicits somatic action potentials from VGN in the attached ganglion (Fig. 3). Vagal afferents with fiber CV excess of 10 m/s and narrow (<1.0 ms) somatic action potentials with upstroke and downstroke velocities in excess of 200 mV/ms and −150 mV/ms, respectively, are classified as myelinated A-type VGN. Those with fiber CV <2 m/s and broad (> 2.0 ms) somatic action potentials with upstroke and downstroke velocities generally less than 55 and −30 mV/ms, respectively are classified as unmyelinated C-type VGN. Bath application of 100 nM ChTX has no measurable effect on the shape of nerve evoked A-type action potentials (Fig. 3A) or the CV of myelinated vagal afferent fibers, i.e., 13.8 ± 3.3 m/s before and 13.8 ± 3.7 m/s after ChTX (n = 5; P > 0.5). Bath application of 100 nM ChTX increases the average duration of nerve evoked C-type action potentials from 2.5 ± 0.5 to 3.0 ± 0.7 ms (Fig. 3B; n = 9; P < 0.05). Interestingly, loss of the BK-type KCa current has no significant effect on the CV of unmyelinated vagal afferent fibers even though the intact vagus nerve is continuously exposed to ChTX, i.e., 0.65 ± 0.8 m/s before and 0.64 ± 0.8 m/s after application of ChTX (n = 9; P > 0.5).
Fig. 3.
Differential effects of charybdotoxin (ChTX) on action potential wave shape using an intact vagal ganglion preparation. Somatic action potentials were evoked through electrical stimulation (▾) of the attached vagus nerve in a myelinated A-type afferent with a conduction velocity (CV) of 11.0 m/s (A) and an unmyelinated C-type afferent with a CV of 0.7 m/s (B). No change in CV, resting membrane potential (RMP), upstroke velocity (UV), and other measures of the action potential wave shape was observed between the control (CNTRL) and ChTX recordings. Differential sensitivity to 100 nM ChTX bath applied to the intact ganglion was most evident in measures of the duration of the nerve evoked action potential duration (APD50; n = 9; *P < 0.05).
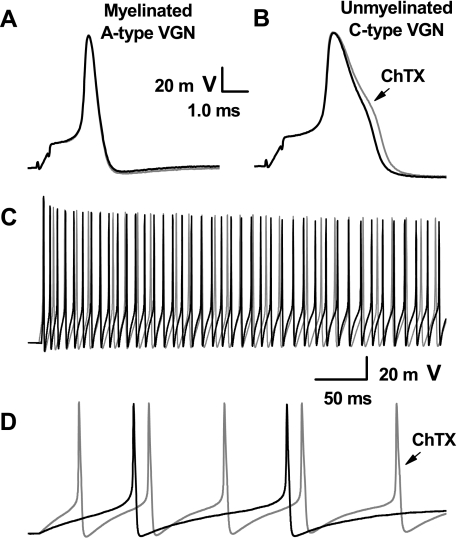
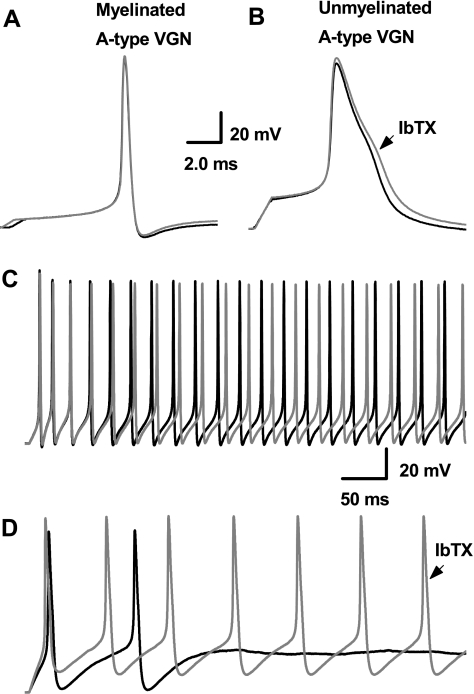
Somatic excitability of VGN is investigated using a high intensity, short duration (500 μs) current pulse and a low intensity, long duration (500–1,000 ms) current step delivered through the patch electrode. Generally pulse magnitudes less than 1 nA elicit a somatic action potentials from A-type VGN with voltage trajectories quite similar to those evoked using nerve stimulation (Fig. 4A). Low magnitude step currents of 150 pA consistently elicit sustained, high-frequency repetitive discharge from all myelinated A-type vagal afferent neurons (44.5 ± 7 Hz; Fig. 4C). Application of 100 nM ChTX has no measurable effect on the shape of the A-type action potential nor is repetitive discharge significantly altered (46.0 ± 7 Hz; P > 0.5; n = 5). In contrast, C-type VGN require pulse magnitudes of 2–3 nA to elicit somatic action potentials but membrane voltage trajectories remain quite similar to those evoked by nerve stimulation (Fig. 4B). High magnitude step currents of 600 pA consistently elicit sustained, low frequency repetitive discharge from all unmyelinated C-type VGN (1.24 ± 0.5 Hz, Fig; 4D). As observed with nerve stimulation, application of 100 nM ChTX brings about a significant broadening of the evoked somatic action potential from 2.60 ± 0.6 to 3.09 ± 0.7 ms (P < 0.05; n = 9). In the presence of the KCa channel antagonist, the same step current magnitude evokes a significantly higher rate of repetitive discharge in C-type VGN (10.8 ± 4 Hz; P < 0.01; n = 9). A summary of the impact ChTX has on the trajectory of membrane voltage and excitability of myelinated A-type and unmyelinated C-type vagal afferents is provided in Table 1.
Fig. 4.
Differential effects of ChTX on neuronal excitability using an intact vagal ganglion preparation. With the use of CV as a definitive measure of afferent type, somatic action potentials were elicited by brief current stimulation through the patch electrode. Again, myelinated A-type afferents (A) were unchanged, while action potentials from unmyelinated C-type afferents (B) broadened in response to 100 nM ChTX. Step depolarizing currents elicited repetitive discharge in both types of afferents, but in contrast myelinated A-type (C) showed no significant difference in discharge frequency while unmyelinated C-type afferents (D) were markedly more excitable in the presence of 100 nM ChTX (gray traces). See Table 1 for summary data.
Table 1.
Impact of 100 nM ChTX and 100 nM IbTx on AP waveform characteristics of VGN identified as myelinated A-type and unmyelinated C-type neurons
| Isolated, 5- to 9-day-old pup |
Intact Ganglia, adult male >250 g |
Intact Ganglia, adult male >250 g |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A type (n = 5) |
C type (n = 6) |
A type (n = 5) |
C type (n = 9) |
A type (n = 6) |
C type (n = 7) |
|||||||
| Rat VGN | CNTRL | ChTX | CNTRL | ChTX | CNTRL | ChTX | CNTRL | ChTX | CNTRL | IbTx | CNTRL | IbTx |
| RMP | −61.8 ± 3 | −59.8 ± 3 | −65.5 ± 5 | −58.6 ± 6* | −62.6 ± 2 | −61.8 ± 1 | −65.9 ± 5 | −66.1 ± 5 | −63.8 ± 2 | −63.2 ± 2 | −64.4 ± 4 | −63.6 ± 4 |
| APFT | −42.1 ± 3 | −41.6 ± 2 | −27.2 ± 3 | −27.4 ± 2 | −44.1 ± 2 | −44.5 ± 2 | −27.5 ± 3 | −27.2 ± 4 | −45.8 ± 5 | −46.1 ± 5 | −28.0 ± 5 | −28.3 ± 5 |
| APFF | 40.6 ± 8 | 41.2 ± 7 | 1.25 ± 0.4 | 11.2 ± 5† | 44.5 ± 7 | 46.0 ± 10 | 1.24 ± 0.5 | 10.8 ± 4† | 47.2 ± 12 | 46.3 ± 11 | 1.57 ± 0.8 | 11.6 ± 4† |
| APD50 | 0.89 ± 0.1 | 0.90 ± 0.1 | 2.54 ± 0.6 | 3.25 ± 0.5† | 0.74 ± 0.2 | 0.76 ± 0.2 | 2.6 ± 0.6 | 3.09 ± 0.7† | 0.78 ± 0.2 | 0.79 ± 0.2 | 2.84 ± 0.5 | 3.34 ± 0.5† |
| APPEAK | 40.6 ± 3 | 40.9 ± 5 | 45.3 ± 3 | 47.9 ± 3* | 48.9 ± 2 | 49.1 ± 4 | 56.2 ± 3 | 55.5 ± 4 | 50.8 ± 6 | 52.0 ± 5 | 53.1 ± 6 | 54.1 ± 5 |
| AHP80 | 18.4 ± 5 | 17.7 ± 5 | 75.7 ± 23 | 73.1 ± 18 | 23.1 ± 15 | 18.6 ± 9 | 83.0 ± 33 | 79.1 ± 24 | 22.8 ± 8 | 21.0 ± 8 | 87.8 ± 23 | 84.7 ± 20 |
| AHPPEAK | −65.8 ± 2 | −66.9 ± 1 | −73.4 ± 2 | −71.7 ± 2 | −67.4 ± 3 | −67.7 ± 3 | −68.8 ± 2 | −69.5 ± 4 | −66.0 ± 2 | −65.5 ± 2 | −66.7 ± 2 | −65.5 ± 1 |
| UVAPD50 | 202.0 ± 33 | 215.0 ± 43 | 47.9 ± 12 | 57.3 ± 17* | 244.0 ± 47 | 241.0 ± 54 | 57.1 ± 12 | 61.5 ± 15 | 233 ± 22 | 232 ± 26 | 56.0 ± 13 | 56.9 ± 11 |
| DVAPD50 | −81.1 ± 7 | −82.5 ± 10 | −33.7 ± 8 | −18.5 ± 5† | −178.0 ± 25 | −177.0 ± 29 | −31.9 ± 9 | −25.7 ± 11† | −181 ± 22 | −179 ± 21 | −31.0 ± 8 | −25.4 ± 8† |
Data are means ± SD. ChTX, charybdotoxin; IbTX, iberiotoxin; AP, action potential; VGN, vagal ganglion neurons; CNTRL, control; RMP, resting membrane potential (mV); APFT, AP firing threshold (mV); APFF, AP firing frequency (Hz), C-type 600 pA and A-type 300 pA step currents; APD50, AP duration at 50% deflection (ms); APPEAK, AP peak deflection (mV); AHPPEAK, peak after hyperpolarization (mV); AHP80, 80% of recovery time to RMP after AHPPEAK (ms); UVAPD50, upstroke velocity at APD50 (mV/ms); DVAPD50, downstroke velocity at APD50 (mV/ms).
P < 0.05,
P < 0.01 vs. CNTRL.
Differential effects of IbTX on action potential discharge of A- and C-type VGN from adult male rat.
Iberiotoxin is a potent and highly selective blocker of the BK-type KCa channel molecularly identified as KCa1.1. While ChTX antagonizes this channel protein, it has also been shown to block an intermediate KCa conductance (KCa3.1) as well as a few subtypes of the Kv1 family of voltage-gated K+ channels (21, 49). To further refine our understanding of the differences in K+ channel subtypes expressed in myelinated and unmyelinated vagal afferents, the current-clamp protocols associated with Figs. 3 and 4 are repeated but with the application of 100 nM IbTX (Fig. 5). Current pulses of the same magnitudes used previously elicit A- and C-type somatic action potentials with membrane voltage trajectories quite similar to those evoked through nerve stimulation (Figs. 4, A and B). Likewise, step currents of the same magnitudes used previously elicit comparable high frequency repetitive discharge in A-type (47.2 ± 12 Hz) and low frequency repetitive discharge in C-type (1.57 ± 0.8 Hz) VGN. As with ChTX, 100 nM IbTX has no effect on the action potential or excitability of A-type VGN (Fig. 4C). Likewise, IbTX broadens the action potentials of unmyelinated C-type VGN to the same extent as that observed with ChTX, 18 vs. 19% (Table 1) and also increased the rate of repetitive discharge by nearly an order of magnitude to 11.6 ± 4 Hz (P < 0.01; n = 7).
Fig. 5.
Differential effects of iberiotoxin (IbTX) on neuronal excitability using an intact vagal ganglion preparation. With the use of CV as a definitive measure of afferent type, somatic action potentials were elicited by brief current stimulation through the patch electrode. Application of 100 nM of IbTX had no effect on myelinated A-type VGN (A), while in contrast action potentials from unmyelinated C-type VGN (B) broadened much in the same manner as with application of 100 nM ChTX. In response to step depolarizing currents, the repetitive discharge characteristics of myelinated A-type afferents (C) were unchanged, while unmyelinated C-type afferents (D) were markedly more excitable in the presence of 100 nM IbTX (gray traces). See Table 1 for summary data.
Differential effects of ChTX on action potential discharge of A- and C-type VGN from neonatal rat.
To determine if the afferent-specific effectiveness of the BK-type KCa channel antagonists was age related comparable electrophysiological studies were carried out in VGN isolated from neonatal rats (5- to 9-days-old). In response to a brief (500 μs) current pulse delivered through the patch electrode, VGN classified as myelinated A-type exhibited low threshold (−42.1 ± 3 mV), brief duration (0.89 ± 0.1 ms) somatic action potentials (Fig. 6A; n = 5). Depolarizing step current injections of 300 pA consistently evoked stable, high frequency action potential discharge (40.6 ± 8 Hz). All measures of action potential discharge characteristics were similar to those from adult A-type neurons albeit somewhat less robust, e.g., measures of upstroke velocity and downstroke velocity of A-type VGN from neonatal rat were somewhat slower than those from adult rat (Table 1). As in the adult preparations, application of 100 nM ChTX did not bring about any significant changes across a comprehensive assessment of action potential wave shape dynamics and repetitive discharge characteristics (Table 1). Consistent with recordings from adult rats, VGN from neonatal rat classified as unmyelinated C-type exhibited much higher threshold for discharge (−27.2 ± 3 mV) and broad duration (2.54 ± 0.6 ms) somatic action potentials with a prominent hump over the time course of repolarization (Fig. 6B; n = 6). Much larger magnitude depolarizing step current injections of 600 pA were required to evoke repetitive action potential discharge, albeit at a much lower average frequency of 1.25 ± 0.4 Hz. Unlike myelinated A-type VGN, all unmyelinated C-type VGN exhibited a number of distinct changes in wave shape and excitability when exposed to 100 nM ChTX (Table 1). Most notable was a nearly 30% increase in the action potential duration from 2.54 ± 0.6 to 3.25 ± 0.5 ms (P < 0.01) along with a concomitant decrease in the downstroke velocity from −33.7 ± 8 to −18.5 ± 5 mV/ms (P < 0.01). Loss of the BK-type KCa current also resulted in a significant increase in the action potential upstroke velocity from 47.9 ± 12 to 57.3 ± 17 mV/ms (P < 0.05), which resulted in a significant increase in the peak amplitude of the action potential from 45.3 ± 3 to 47.9 ± 3 mV (P < 0.05). Collectively, these changes led to a substantial increase in excitability of unmyelinated C-type VGN, as the same 600-pA step current magnitude now elicited sustained repetitive discharge frequencies nearly 10 times faster than control (11.2 ± 4.7 vs. 1.25 ± 0.4 Hz; P < 0.01), an effect that closely paralleled those ChTX-mediated changes in excitability observed using an adult preparation. However, RMP, upstroke velocity (UVAPD50), and action potential peak voltage (APPEAK) were all significantly altered in the presence of ChTX in C-type (but not A-type) neonatal VGN (Table 1). These effects were not observed in unmyelinated C-type VGN from adult rats. A comparison of the pooled measures of action potential discharge (Table 1) suggests such subtle functional differences may be the consequence of as yet unrecognized neurobiological factors in the developmental expression of the voltage- and/or Ca+2-dependent properties of the BK-type KCa channel.
Fig. 6.
Differential effects of ChTX on action potential discharge of neonatal rat VGN. A: somatic action potentials elicited from a neonatal rat VGN identified as myelinated A-type before (CNTRL, black line) and after (ChTX, gray line) application of 100 nM ChTX. Bar plots and step depolarizations summarize the lack of an effect on the excitability of myelinated A-type VGN. B: somatic action potentials elicited from an unmyelinated C-type VGN from neonatal rat under the same control (CNTRL) and test (ChTX) conditions as in A. Bar plots and step depolarizations summarize the dramatic effect 100 nM ChTX has on the excitability of unmyelinated C-type VGN. See text and Table 1 for additional details. *P < 0.05; **P < 0.01.
Differential effect of ChTX on the whole cell K+ current of A- and C-type VGN from neonatal rat.
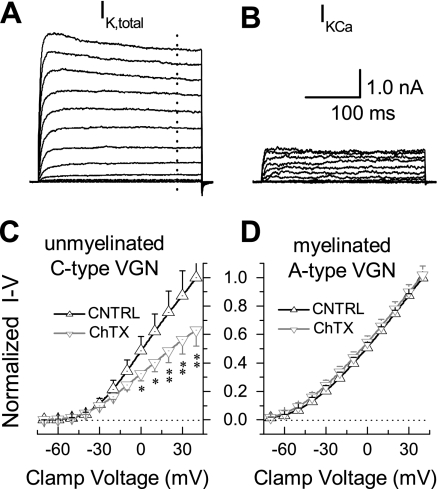
Given the difference in the effectiveness of the BK-type KCa channel antagonists between myelinated and unmyelinated vagal afferents, a voltage-clamp study was carried out to determine if myelinated VGN lacked a significant BK-type KCa current. An initial current-clamp recording was carried out using the extracellular action potential recording solution to reliably classify the cell under study as a myelinated or unmyelinated VGN (28). The extracellular solution was then exchanged for one suitable for recording whole cell K+ currents. From a holding potential of −80-mV, 400-ms voltage-clamp steps were applied in increments of +10 mV to a maximum of +40 mV. This protocol elicited a rapidly activating and sustained outward K+ current (Fig. 7A). This protocol was repeated in the presence of 100 nM ChTX, which resulted in a marked reduction in the whole cell K+ current. When these traces were digitally subtracted from the control traces, the voltage- and time-dependent profile of the ChTX-sensitive K+ current was revealed to have comprised ∼25% of the whole cell K+ current under control conditions (Fig. 7B). The normalized current-voltage (I-V) profiles from a sample (n = 8) of VGN identified unmyelinated afferents showed that ChTX significantly reduced the outward whole cell K+ current at voltage-clamp steps to 0 mV and above (Fig. 7C). Consistent with the action potential recordings from adult rats, 100 nM ChTX had no measurable effect on the normalized I-V profiles from VGN-identified myelinated afferents (n = 7; Fig. 7D).
Fig. 7.
Voltage-clamp evidence for differential expression of a BK-type KCa current. A: from a cell classified as an unmyelinated C-type VGN, depolarizing voltage-clamp steps revealed a large, sustained outward K+ current (IK,total). B: K+ current eliminated with the application of 100 nM ChTX (subtracted records, see text). For A and B, the activation protocol consisted of 300-ms voltage-clamp steps at 3-s intervals from a Vhold of −80 mV to +40 mV in increments of +10 mV. C: normalized current-voltage (I-V) relationship for the whole cell K+ currents from cells classified as unmyelinated C-type VGN under control (CNTRL, n = 8) conditions and in the presence of 100 nM ChTX. D: normalized I-V relationship for the whole cell K+ current from cells classified as a myelinated A-type VGN under control (CNTRL, n = 7) conditions and in the presence of 100 nM ChTX. For C and D, data are means ± SD of measures made 250 ms into the clamp step (dotted trace, Fig. 7A) with *P < 0.05 and **P < 0.01 vs. CNTRL.
DISCUSSION
Electrophysiological, pharmacological, and molecular biological studies have documented the functional contribution of a wide variety of ion channels in vagal afferent neurophysiology (47). Of the candidate K+ ion channels functionally expressed in vagal neurons, nanomolar concentrations of ChTX block the large conductance BK-type KCa1.1 and the Kv1.2 channel subtypes (17, 18, 20, 22). Nanomolar concentrations of ChTX can also block the margatoxin-sensitive Kv1.3 and the maurotoxin-sensitive intermediate-conductance KCa3.1 channel subtypes (20, 50). However, the nearly identical effects of ChTX and IbTX on the action potential waveform and excitability of unmyelinated C-type afferents suggest that the functional consequences of 100 nM ChTX on the whole cell K+ current in adult male rats is effectively limited to blocking of the BK-type KCa1.1 channel. If ChTX is also blocking Kv1.2, Kv1.3, or a KCa3.1 channel current, the functional contribution of the particular K+ channel subtype must be below the threshold for detection using our current-clamp protocols.
It is possible that the disparate action of ChTX across myelinated and unmyelinated vagal afferents might also suggest a bimodal distribution of Kv1.2 in addition to KCa1.1, but there are contradictory lines of evidence in the literature. A study combining RT-PCR and patch-clamp electrophysiology suggests that an α-dendrotoxin (α-DTX; 10–100 nM)-sensitive component of the whole cell K+ current (IDTX) in rat vagal neurons was comprised of the Kv1.1, Kv1.2, and Kv1.6 channel subtypes in the majority of presumably myelinated A- and unmyelinated C-type afferents (18). In the accompanying current-clamp experiments reported by Glazebrook et al. (18), α-DTX had no effect on resting membrane potential, modest effects on the amplitude and duration of the action potential, and prominent effects on excitability of both A- and C-type vagal afferents from both neonatal and adult rat. Apart from the elevation in excitability of C-type neurons, these three general experimental observations are contrary to our own (Figs. 3–6 and Table 1) along with some interesting differences between neonatal and adult rats (see below). At present, the relative contribution of the Kv1.1, Kv1.2, and Kv1.6 channel subtypes to the total IDTX in rat is unknown. Assuming that Kv1.2 is functionally expressed in myelinated A-type neurons, then 100 nM ChTX should be sufficient to block its contribution to the whole cell K+ current (20). However, we measured no statistical differences across a wide range of functional measures of sustained neuronal excitability, action potential wave shape, and voltage dependence of the whole cell K+ current from rat myelinated A-type neurons in the presence of 100 nM ChTX (Table 1). One possible explanation is that the mRNA for Kv1.2 observed in presumably myelinated A-type neurons does not translate into functional membrane bound ion channels in myelinated afferent neurons of healthy rats. Or perhaps alternative current-clamp protocols are necessary to reveal a role for the Kv1.2 channel current in the excitability of myelinated A-type vagal afferents. These suppositions need to be validated using highly selective antagonists for Kv1.2 in conjunction with experimental validation of afferent fiber type (27, 28, 36). In that regard, companion studies could also resolve the potential role of Kv1.2 in unmyelinated C-type vagal neurons. At present the extent to which ChTX blocking of Kv1.2 in C-type neurons contributed to the increase in action potential duration and excitability is unknown. However, the negative effects of α-DTX on resting membrane potential (neonatal rat) and modest impact on action potential duration and peak height (neonatal rat) (18) stand in stark contrast to our observations of the effects of the Kv1.2 channel blocker ChTX on unmyelinated C-type vagal afferents (Figs. 2, 4, and 5; Table 1). Furthermore, there is no evidence in the afferent literature that loss of the Kv1.2 current brings about significant action potential broadening of sensory afferent neurons, while the BK-type KCa channel current has long been implicated in such a role (14, 29, 41).
KCa1.1 and the potential for a differential distribution of modulatory β-subunits.
The combined results of our immunohistochemical studies (Figs. 1 and 2) and electrophysiological recordings documenting the afferent-specific effectiveness of ChTX and IbTX (Figs. 3–7) are suggestive of a marked functional expression of KCa1.1 in unmyelinated C-type VGN with little to no functional expression of this channel subtype in myelinated A-type VGN. However, KCa1.1 channels are known to associate with four different modulatory β-subunits (β1-4; KCNMB1–4) (51). One of these subunits, β4, can render the calcium-activated potassium channel subunit α1 (KCa1.1) resistant to nanomolar concentrations of ChTX and IbTX (33). It is plausible to consider that the complete insensitivity of A-type VGN to the effects of ChTX and IbTX comes about through an association of KCa1.1 with the β4-subunit. Such a hypothesis implies that the subunit compositions of the BK channels in unmyelinated and myelinated afferents may be different. This interpretation would be consistent with recent studies showing that the heterogeneity in BKCa current function in cutaneous neurons may result from, at least in part, a differential distribution of β-subunits (55). Indole-diterpenes such as paxilline can inhibit the IbTX-insensitive β4-subunit isoform of the KCa1.1 channel (25). Therefore, further investigation using paxilline as a selective antagonist of KCa1.1 may provide a means to clarify and add support to the conclusions presented in this study.
Literature support for a differential expression of KCa1.1 across vagal sensory afferent terminals.
It has long been recognized that unmyelinated vagal afferents exhibit a far broader sensitivity to physiologically relevant chemicals such as inflammatory mediators than myelinated vagal afferents (23). The physiological implications of this fact remain somewhat elusive. However, recent results from intact in vitro and in situ preparations are beginning to demonstrate that KCa channels and in particular the BK-type KCa1.1 generally are not functionally involved in the process of sensory reception at the peripheral afferent terminal ending of mechanosensitive myelinated vagal afferents although some exceptions can be noted for particular species and organ systems (16, 32, 37, 54). Much less is known concerning the ion channel subtypes providing a functional contribution to the sensory encoding process at the peripheral terminals of unmyelinated vagal afferents due, in large part, to recording difficulties associated with these fragile and small diameter fibers. However, there are intriguing data from airway afferent preparations that BK-type KCa channels may play an important role in prejunctional modulation of neuropeptides released from unmyelinated peripheral terminals, the implication being that BK-type KCa channels may play a critically important role in the response of sensory nerve terminals to neurogenic inflammation in addition to the more widely recognized impact on discharge threshold through modulation of resting membrane potential (10, 11, 44, 45, 52, 53).
Reynolds et al. (37) used an in vitro rat aortic arch preparation for recording the pressure-dependent discharge of single, identified myelinated baroreceptor fibers. Neither intralumenal exposure of 100 nM ChTX for up to 30 min nor direct application of the drug to the adventitial tissue area pervaded by the afferent terminal endings altered the pressure encoding characteristics of myelinated baroreceptors (37). Single fiber recordings of low threshold, myelinated vagal mechanoreceptors and the effects of BK-type KCa antagonists have also been carried out utilizing esophageal and airway in vitro preparations most often from guinea pig. Zagorodnyuk et al. (54) demonstrated that 100 nM of iberiotoxin, a potent and selective antagonist for KCa1.1, failed to alter stretch-evoked firing. Interestingly, 100 nM ChTX did produce a modest increase in spontaneous and stretch-evoked firing of myelinated esophageal afferents but obviously not as a result of KCa1.1 blockade. This is an observation supported by others (32, 54) and perhaps suggests that low threshold mechanoreceptors of guinea pig esophagus and airway, perhaps unlike the rat, functionally expressed Kv1.2 channels at the peripheral termination of myelinated mechanosensory afferents along with other subtypes of dendrotoxin-sensitive ion channels.
Nociceptive and mechanosensitive unmyelinated vagal afferents prominently participate in centrally mediated reflexes in addition to releasing neuropeptides at their peripheral terminals, playing an important role in mediating localized neurogenic inflammation and afferent sensitization throughout the viscera (3, 7, 10, 12, 45). Studies investigating the potential contribution that KCa1.1 channels may make to the neural encoding of arterial pressure by unmyelinated rat aortic baroreceptors have yet to be carried out. However, there are numerous autocrine and paracrine chemical factors known to impact the sensitivity of unmyelinated baroreceptors that can also have modulatory effects on BK-type KCa channel gating both through intracellular signaling molecules and pathways (9, 13). Far more direct evidence of a role for BK-type KCa channels in mediating unmyelinated afferent function is available from studies of guinea pig airway afferents. It has long been recognized that ChTX-sensitive KCa channels exhibit the capacity for prejunctional modulation of the release of peptides from capsaicin-sensitive unmyelinated airway afferents (35, 44). More recently, Yoshihara et al. (52, 53) have demonstrated a similar role for ChTX-sensitive KCa channels in mediating the effects of cannabinoid and neuroactive steroid receptor agonists in unmyelinated guinea pig airway afferents.
Conclusions.
This study provides compelling evidence that a BK-type KCa current makes a functional contribution to the neurophysiological properties of unmyelinated but not myelinated vagal afferent neurons from healthy rats. Labeling for IB4-containing neurons that are presumably mostly unmyelinated C-type afferents significantly coexpressed with markers for the BK-type KCa1.1 channel protein (KCNMA1). The implication being that those cells strongly expressing the BK-type KCa1.1 channel protein are presumably mostly unmyelinated C-type afferents (Figs. 1 and 2). Current and voltage-clamp recordings documenting the effects of ChTX and IbTX on action potential discharge and the whole cell K+ current were examined using rat vagal afferent neurons of known fiber type (28). In both neonatal (P5–9) and adult male (>250 g) rat preparations, neither 100 nM ChTX nor 100 nM IbTX had any effects on numerous measures of neuronal discharge and whole cell K+ current I-V characteristics in myelinated A-type neurons, while the same antagonist concentration consistently broadened somatic action potentials, increased neuronal excitability, and decreased the whole cell K+ current in unmyelinated C-type vagal neurons (Figs. 3–7 and Table 1).
Advances in the understanding of the molecular structure and chemical sensitivities of BK-type KCa channels have resulted in pharmacological agents that offer considerable potential as therapeutic interventions for pathophysiological conditions of the central nervous system and pulmonary afferents (19, 45). Much less attention has been directed toward understanding the role of BK-type KCa channels in other areas of visceral sensory afferent function. However, recent investigations surrounding the selective modulation of BK-type KCa channels have shown the potential for novel pharmacological treatments for a wide range of visceral organ system diseases (42, 43). Collectively, our results are highly suggestive of a preferential expression of the KCa1.1 channel in unmyelinated but not myelinated vagal afferents of male rat. Given the vastly different neurophysiological properties and reflexogenic capacities of these distinct classes of sensory afferents, such a biophysical dichotomy would greatly enhance the potential for fiber-specific manipulation of autonomic nervous system function and dysfunction.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-072012 and HL-081819 (to J. H. Schild) and HL-061436 (to D. L. Kunze).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the reviewers for a very careful reading of our manuscript and in particular reviewer #2 for providing insightful comments concerning a potential role for modulatory β-subunits in the interpretation of our results.
REFERENCES
- 1. Bahia PK, Suzuki R, Benton DC, Jowett AJ, Chen MX, Trezise DJ, Dickenson AH, Moss GW. A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J Neurosci 25: 3489–3498, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnes PJ. The problem of cough and development of novel antitussives. Pulm Pharmacol Ther 20: 416–422, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19: 1–19, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Boettger MK, Till S, Chen MX, Anand U, Otto WR, Plumpton C, Trezise DJ, Tate SN, Bountra C, Coward K, Birch R, Anand P. Calcium-activated potassium channel SK1- and IK1-like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain 125: 252–263, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Browning KN, Mendelowitz D. Musings on the wanderer: what's new in our understanding of vago-vagal reflexes?: II. Integration of afferent signaling from the viscera by the nodose ganglia. Am J Physiol Gastrointest Liver Physiol 284: G8–G14, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bueno L, Fioramonti J. Effects of inflammatory mediators on gut sensitivity. Can J Gastroenterol 13 Suppl A: 42A–46A, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Buniel M, Glazebrook PA, Ramirez-Navarro A, Kunze DL. Distribution of voltage-gated potassium and hyperpolarization-activated channels in sensory afferent fibers in the rat carotid body. J Comp Neurol 510: 367–377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calderone V. Large-conductance, Ca(2+)-activated K(+) channels: function, pharmacology and drugs. Curr Med Chem 9: 1385–1395, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Carr MJ, Undem BJ. Inflammation-induced plasticity of the afferent innervation of the airways. Environ Health Perspect 109 Suppl 4: 567–571, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carr MJ, Undem BJ. Ion channels in airway afferent neurons. Respir Physiol 125: 83–97, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol 61: 45–54, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann NY Acad Sci 940: 1–19, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Christian EP, Togo J, Naper KE. Guinea pig visceral C-fiber neurons are diverse with respect to the K+ currents involved in action-potential repolarization. J Neurophysiol 71: 561–574, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol Heart Circ Physiol 275: H632–H640, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Fox AJ, Barnes PJ, Venkatesan P, Belvisi MG. Activation of large conductance potassium channels inhibits the afferent and efferent function of airway sensory nerves in the guinea pig. J Clin Invest 99: 513–519, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia ML, Knaus HG, Munujos P, Slaughter RS, Kaczorowski GJ. Charybdotoxin and its effects on potassium channels. Am J Physiol Cell Physiol 269: C1–C10, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Glazebrook PA, Ramirez AN, Schild JH, Shieh CC, Doan T, Wible BA, Kunze DL. Potassium channels Kv1.1, Kv1.2 Kv1.6. influence excitability of rat visceral sensory neurons. J Physiol 541: 467–482, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gribkoff VK, Starrett JE., Jr and Dworetzky SI. Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist 7: 166–177, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 12, 13, 15, and 31, stably expressed in mammalian cell lines. Mol Pharmacol 45: 1227–1234, 1994 [PubMed] [Google Scholar]
- 21. Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57: 473–508, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Hay M, Kunze DL. Calcium-activated potassium channels in rat visceral sensory afferents. Brain Res 639: 333–336, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Higashi H. Pharmacological aspects of visceral sensory receptors. Prog Brain Res 67: 149–162, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Hoesch RE, Weinreich D, Kao JP. Localized IP3-evoked Ca2+ release activates a K+ current in primary vagal sensory neurons. J Neurophysiol 91: 2344–2352, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci 21: 9585–9597, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li BY, Feng B, Tsu HY, Schild JH. Unmyelinated visceral afferents exhibit frequency dependent action potential broadening while myelinated visceral afferents do not. Neurosci Lett 421: 62–66, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Li BY, Schild JH. Patch clamp electrophysiology in nodose ganglia of adult rat. J Neurosci Meth 115: 157–167, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Meth 164: 75–85, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li W, Gao SB, Lv CX, Wu Y, Guo ZH, Ding JP, Xu T. Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J Cell Physiol 212: 348–357, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Li Z, Lee HC, Bielefeldt K, Chapleau MW, Abboud FM. The prostacyclin analogue carbacyclin inhibits Ca(2+)-activated K+ current in aortic baroreceptor neurones of rats. J Physiol 501: 275–287, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McAlexander MA, Undem BJ. Potassium channel blockade induces action potential generation in guinea-pig airway vagal afferent neurones. J Auton Nerv Syst 78: 158–164, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience 48: 501–524, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Miura M, Belvisi MG, Stretton CD, Yacoub MH, Barnes PJ. Role of K+ channels in the modulation of cholinergic neural responses in guinea-pig and human airways. J Physiol 455: 1–15, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pimentel C, M'Barek S, Visan V, Grissmer S, Sampieri F, Sabatier JM, Darbon H, Fajloun Z. Chemical synthesis and 1H-NMR 3D structure determination of AgTx2-MTX chimera, a new potential blocker for Kv1.2 channel, derived from MTX and AgTx2 scorpion toxins. Protein Sci 17: 107–118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reynolds PJ, Yang M, Andresen MC. Contribution of potassium channels to the discharge properties of rat aortic baroreceptor sensor endings. Brain Res Bull 665: 115–122, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Santiago S, Ferrer T, Espinosa ML. Neurophysiological studies of thin myelinated (A delta) and unmyelinated (C) fibers: application to peripheral neuropathies. Neurophysiol Clin 30: 27–42, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Schild JH, Alfrey KD, Li BY. Voltage-gated ion channels in vagal afferent neurons. In: Advances in Vagal Afferent Neurobiology, edited by Undem BJ, Weinreich D. Boca Raton, FL: CRC, 2005, p. 77–100 [Google Scholar]
- 40. Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A- and C-type rat nodose sensory neurons: model interpretations of dynamic discharge characteristics. J Neurophysiol 71: 2338–2358, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Scholz A, Gruss M, Vogel W. Properties and functions of calcuim-activated K+ channels in small neurons of rat dorsal root ganglion studied in a thin slice preparation. J Physiol 513: 55–69, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sivarao DV, Newberry K, Langdon S, Lee AV, Hewawasam P, Plym MJ, Signor L, Myers R, Lodge NJ. Effect of 4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl)-6-(trifluoromethyl)-quinol in-2(1H)-one (BMS-223131), a novel opener of large conductance Ca2+-activated K+ (maxi-K) channels on normal and stress-aggravated colonic motility and visceral nociception. J Pharmacol Exp Ther 313: 840–847, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Snitsarev V, Whiteis CA, Chapleau MW, Abboud FM. Mechano- and chemosensitivity of rat nodose neurones–selective excitatory effects of prostacyclin. J Physiol 582: 177–194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stretton D, Miura M, Belvisi MG, Barnes PJ. Calcium-activated potassium channels mediate prejunctional inhibition of peripheral sensory nerves. Proc Natl Acad Sci USA 89: 1325–1329, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Undem BJ, Carr MJ. Pharmacology of airway afferent nerve activity. Respir Res 2: 234–244, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Undem BJ, Kollarik M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 355–360, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Undem BJ, Weinreich D. Advances in Vagal Afferent Neurobiology. Boca Raton, FL: CRC, 2005 [Google Scholar]
- 48. Wang Y, Wang DH. Neural control of blood pressure: focusing on capsaicin-sensitive sensory nerves. Cardiovasc Hematol Disord Drug Targets 7: 37–46, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57: 463–472, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97: 8151–8156, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wulff H, Zhorov BS. K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem Rev 108: 1744–1773, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoshihara S, Morimoto H, Ohori M, Yamada Y, Abe T, Arisaka O. A neuroactive steroid inhibits guinea pig airway sensory nerves via Maxi-K channel activation. Int Arch Allergy Immunol 141: 31–36, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Yoshihara S, Morimoto H, Yamada Y, Abe T, Arisaka O. Cannabinoid receptor agonists inhibit sensory nerve activation in guinea pig airways. Am J Respir Crit Care Med 170: 941–946, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. 4-aminopyridine- and dendrotoxin-sensitive potassium channels influence excitability of vagal mechano-sensitive endings in guinea-pig oesophagus. Br J Pharmacol 137: 1195–1206, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang XL, Mok LP, Katz EJ, Gold MS. BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. Eur J Neurosci 31: 450–462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]