Fig. 1.
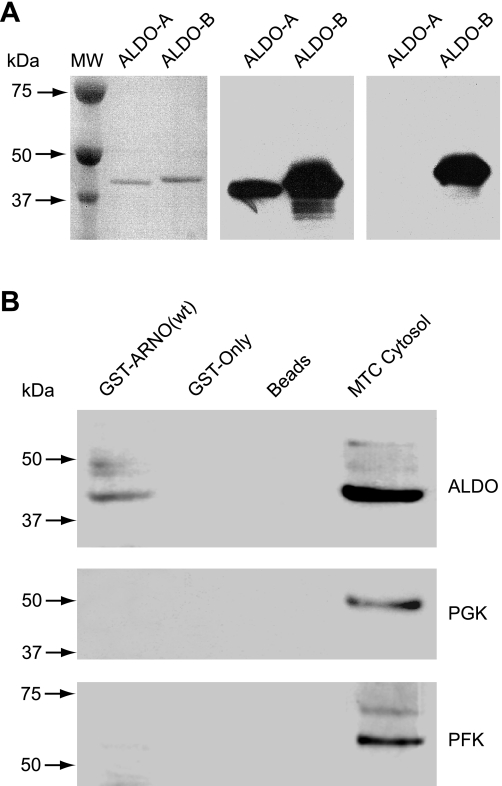
Cytosolic aldolase specifically interacts with ADP-ribosylation factor nucleotide site opener (ARNO) in pull-down experiments. A: evaluation of specificity of anti-aldolase antibodies used in this study. Left image shows 1 μg of aldolase-A (ALDO-A) from rabbit muscle and 1 μg of 6His-tagged recombinant human ALDO-B resolved by NuPAGE, transferred to Immobilon membrane, and stained with Ponceau-S. Middle image shows Western blotting performed with the anti-ALDO-A/B (D-18) antibody, which recognizes both ALDO-A and ALDO-B. Right image shows Western blotting performed with anti-ALDO-B (HPA002198) antibody, which selectively recognizes ALDO-B. B: specific interaction of full-length wild-type (wt) ARNO with aldolase but not with other enzymes of the glycolytic pathway. Pull-down with glutathione S-transferase (GST)-ARNO(wt) from cytosolic fractions of cultured mouse proximal tubule cells (MTC) was followed by Western blot analysis using anti-ALDO-A/B (D-18) antibody. There was a specific interaction of ARNO with aldolase but not with 2 other enzymes of the glycolytic pathway, phosphofructokinase (PFK) or phosphoglycerate kinase (PGK).