Abstract
Retinal transplantation experiments have advanced considerably during recent years, but remaining diseased photoreceptor cells in the host retina physically obstruct the development of graft–host neuronal contacts that are required for vision. We here report selective removal of photoreceptors using the biodegradable elastomer poly(glycerol sebacate) (PGS). A 1 × 3 mm PGS membrane was implanted in the subretinal space of normal rabbit eyes, and morphologic specimens were examined with hematoxylin and eosin staining and a panel of immunohistochemical markers. Seven days postoperatively, a patent separation of the neuroretina and retinal pigment epithelium was found as well as loss of several rows of photoreceptors in combination with massive terminal transferase-mediated dUTP nick-end labeling (TUNEL) staining for apoptosis in the outer nuclear layer. After 28 days, the neuroretina was reattached, the PGS membrane had degraded, and photoreceptors were absent in the implantation area. Activated Müller cells were found in the entire retina in 7-day specimens, and in the implantation area after 28 days. AII amacrine and rod bipolar cell morphology was not affected, except for disrupted dendritic branching, which was present in rod bipolar cells in 28-day specimens. We conclude that retinal detachment induced by the biodegradable PGS membrane creates a permissive environment in which graft–host neuronal connections may be facilitated in future retinal transplantation experiments.
Introduction
Retinal transplantation experiments aimed at relieving symptoms in patients suffering from retinal degenerative disease have been ongoing for more than two decades, but to date, transplantation has not transcended into clinical practice. Laboratory experiments have shown that immature full-thickness neuroretinal grafts can develop normal photoreceptors and survive for extended periods without immune suppression in hosts with retinal degeneration.1,2 However, transmission of visual information from the transplant to the host CNS has not been attained due to lack of integration of graft-derived neurons with the host retina. Graft–host integration has been shown sporadically in cases where host photoreceptors are absent, indicating that removal of these cells before actual transplantation may be important.3,4
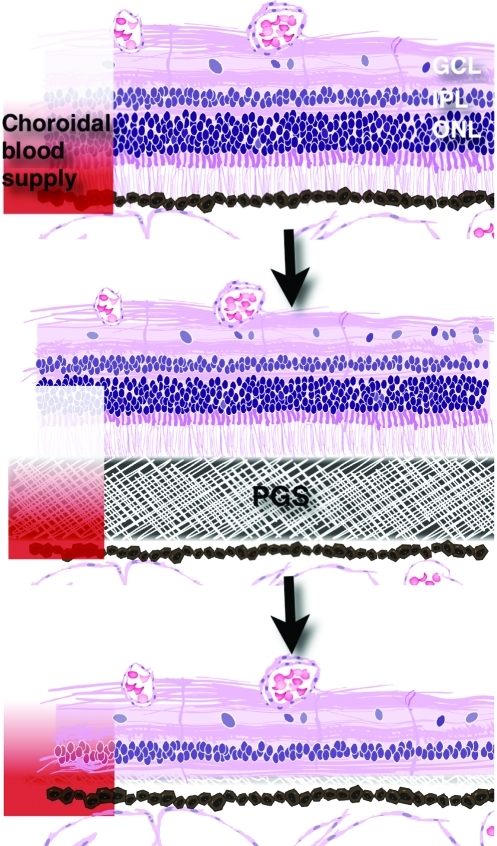
Prolonged separation of the neuroretina from the retinal pigment epithelium (RPE), retinal detachment, leads to degeneration of photoreceptors due to diminished nutritive support from the choroid.5 For the present experiment, we wanted to investigate if photoreceptors can be removed by temporary retinal detachment induced by a biodegradable membrane, poly(glycerol sebacate) (PGS), placed in the subretinal space. The successful use of a biomaterial in this setting would provide a convenient and selective route to eliminate a diseased photoreceptor layer before or in conjunction with transplantation of a new healthy photoreceptor layer (Fig. 1). PGS is a tough elastomeric polymer with mechanical properties similar to those of tissue and vulcanized rubber, which allow mechanical compliance with tissue and enable facile surgical handling of the polymer.6 Especially important for the present application, PGS degrades through a surface erosion process that results in loss of mass with no detectable swelling or change in the geometry of the bulk polymer.7 Recent studies demonstrate the in vitro biocompatibility of PGS with 3T3 human fibroblasts, baboon primary endothelial progenitor cells and smooth muscle cells, rat Schwann cells, and mouse retinal progenitor cells.6,8–10 Additionally, in vivo studies have shown that PGS causes less inflammation and fibrosis than poly(lactide-co-glycolide), a well-studied and widely used biomaterial, and in contrast to poly(lactide-co-glycolide), PGS does not induce a foreign body giant cell response.6,9
FIG. 1.
Theoretical model of isolated photoreceptor removal using the degradable, poly(glycerol sebacate) (PGS) membrane. The membrane disrupts the normal choroidal blood supply to the outer nuclear layer (ONL) causing selective removal of these cells. GCL, ganglion cell layer; IPL, inner plexiform layer. Color images available online at www.liebertonline.com/tea
Several studies report the fabrication of PGS into structures for supporting tissue growth using methods such as salt leaching11 and microfabrication.10,12,13 We recently reported the microfabrication of a thin porous PGS membrane for use as a scaffold in retinal tissue engineering, specifically for photoreceptor replacement using retinal progenitor cells.10 The microfabrication method allows precise customization of the size, shape, and density of the pores in the scaffold, and the pores were included in the design of the scaffold specifically to allow nutrients to flow from the RPE to the photoreceptor layer. For the present study, we reasoned that a similarly fabricated PGS membrane lacking pores would serve to block the flow of nutrients to the photoreceptor layer and thus cause selective removal of the photoreceptor layer.
Materials and Methods
Preparation of PGS membranes
Fabrication of the PGS scaffolds was carried out in a class 10000 clean room. A 10:1 prepolymer:hardener mixture of PDMS (Sylgard 184; Dow Corning, Midland, MI) was outgassed under vacuum, poured into a Petri dish, and cured for 1 h at 60°C. The PDMS slab was removed from the Petri dish and plasma oxidized for 1 min to create a hydrophilic surface.14–16 The top surface was spin coated at 3000 rpm for 30 s with a 61.5% w/v aqueous sucrose solution (0.2 μm filtered) within 5 min of plasma treatment. The sucrose-coated PDMS was immediately baked at 130°C in an oven for 10 min and then transferred to a 120°C hotplate. Approximately 6.5 g of molten PGS (150°C) (prepared as previously described6,10) was spin coated at 3000 rpm for 30 s on the sucrose-coated PDMS slab. The PGS on the PDMS slab was cured at 120°C under a vacuum of 15 millitorr for 48 h. Subsequently, the mold was submerged in ddH2O for 16 days to loosen the PGS from the PDMS slab. The PGS was subsequently peeled off of the PDMS using forceps while keeping the PGS and PDMS submerged in water. The resultant PGS membranes were found to be approximately 45 μm thick by optical microscopy.
The PGS membranes were stored as 10 × 10 mm sheets on a glass slide at 4°C. The glass slide containing the membranes was submerged with the PGS side up into distilled water and allowed to soak for 10 min. The edge of a PGS membrane was carefully grabbed with fine tweezers and peeled off the glass slide. Two hours before surgery, the PGS membranes were soaked in 70% ethanol and then rinsed with phosphate-buffered saline (PBS) under sterile conditions. Just before implantation, 1 × 3 mm strips of membrane was cut using a scalpel. Once the membrane was removed from the PBS, it adopted a slightly folded shape and could be drawn into the glass cannula (see second section under “Surgical procedures”).
Surgical procedures
Five pigmented mixed-strain adult rabbits, aged 4 months, from a local breeder, were used as hosts. Thirty minutes before surgery, 10% phenylephrine (Novartis, Täby, Sweden) and 1% cyclopentolate (Alcon Sverige AB, Stockholm, Sweden) eye drops were instilled in the right eye of the host rabbit for pupil dilation. The rabbit was anesthetized with one part xylazine (20 mg/mL; Bayer, Leverkusen, Germany) and three parts ketamine (50 mg/mL; Pfizer AB, Sollentuna, Sweden) to 1 mL/kg intramuscularly in the thigh. Just before surgery, 0.5% tetracaine eye drops (Novartis) were instilled in the eye.
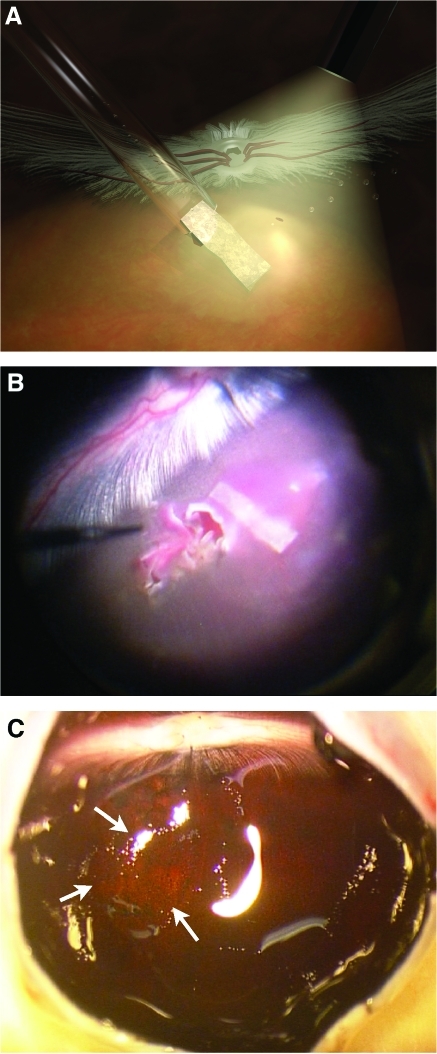
The right eye of each rabbit was used for implantation of the PGS membrane, while the left eye served as a control. The implantation technique largely followed our previously published protocol for full-thickness neuroretinal transplantation.17 Briefly, a core vitrectomy including posterior vitreous detachment was performed using an Alcon Accurus® vitrectomy machine (Alcon Laboratories, Fort Worth, TX). A Zeiss surgical microscope (Carl Zeiss, Oberkochen, Germany) equipped with a BIOM® lens (Oculus, Wetzlar, Germany) was used for visualization of the fundus. After the vitrectomy, a 30-gauge blunt anterior chamber needle (Visitech, Sarasota, FL) attached to a syringe containing Ames' solution was inserted through the sclera into the vitreous cavity under visual guidance. A retinotomy was made approximately 2–3 mm inferior to the optic disc with the tip of the needle, and a retinal detachment bleb formed by infusing Ames' solution into the subretinal space. A second, smaller retinotomy was made in the bleb periphery. The PGS membrane was drawn into a siliconized glass tube, and the tube inserted into the vitreous cavity through the 10 o'clock sclerotomy. Using an attached syringe, the membrane was injected into the bleb through the larger retinotomy, while the excess Ames' solution escaped out of the smaller retinotomy (Fig. 2A). One membrane was used for each host eye. The conjunctiva and sclerotomies were then sutured. Gentamicin (Sigma, St. Louis, MO) 2.5 mg and betamethasone (Swedish Orphan Intl., Stockholm, Sweden) 1 mg were injected subconjunctivally, and chloramphenicol ointment (Pfizer AB) was instilled. The animals were observed closely until awakening.
FIG. 2.
The PGS membrane in the subretinal space of rabbit eyes. (A) Illustration of the PGS implantation procedure. After a core vitrectomy including a posterior vitreous detachment, a local neuroretinal detachment has been created by infusing Ames' solution through a retinotomy and into the subretinal space. A second pinpoint retinotomy has been made in the bleb. The PGS membrane, drawn into a siliconized glass capillary, is introduced into the eye, and pushed out into the subretinal space using fluid from an attached syringe. As the membrane enters the subretinal space, the excess fluid escapes through the second retinotomy. (B) Perioperative photograph through the operating microscope. The membrane can be seen as a gray rectangular shape together with a small amount of blood derived from the choroid. (C) Dissection, 28 days after PGS implantation. The retina is attached, and an area with altered choroidal pigmentation is present (arrows). Color images available online at www.liebertonline.com/tea
No postoperative treatment was given. The fundus of all operated eyes was inspected using a binocular indirect ophthalmoscope 2 days after surgery. All proceedings and animal treatment were in accordance with the guidelines and requirements of the Government Committee on Animal Experimentation at Lund University and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Tissue preparation
The rabbits were sacrificed with 5 mL sodium pentobarbital (60 mg/mL; Apoteksbolaget, Umeå, Sweden) intravenously 7 days (n = 2) or 28 days (n = 3) after surgery. The operated as well as the unoperated eye were enucleated, a cut made through the sclera in the pars plana region, and the eyes put in 0.1 M Sørensen's phosphate buffer at pH 7.4 containing 4% paraformaldehyde for 10–15 min. The cornea, lens, and vitreous were then removed, and the posterior part of the eye again put in the paraformaldehyde buffer for 4 h after which the eyecups were examined and photographed in the operating microscope. Tissue specimens 2–3 mm wide including the implant area and parts of the medullary rays and optic nerve were dissected out. After an initial washing in Sørensen's 0.1 M phosphate buffer at pH 7.4 and repeated washings in Sørensen's buffer with sucrose added in increasing concentrations (10–25%), the specimens were embedded in Yazulla (30% egg albumen and 3% gelatine in water). They were then sectioned on a cryostat at 12 μm and mounted on glass slides. Every 10th slide was stained with hematoxylin and eosin.
For immunohistochemical labeling, the following primary antibodies were used: anti-glial fibrillary acidic protein (GFAP, activated Müller cells) (MAB3402; Chemicon International, Temecula, CA; monoclonal, diluted 1:200); anti-protein kinase C (PKC, rod bipolar cells) (K01107M; Nordic Biosite AB, Täby, Sweden; monoclonal, diluted 1:200); anti-parvalbumin (AII amacrine cells) (Sigma; monoclonal, diluted 1:1000); anti-rhodopsin (rod photoreceptors) (Rho4D2, a kind gift from Prof. R.S. Molday, Vancouver, Canada; monoclonal, diluted 1:100); anti-synaptophysin (presynaptic vesicles) (Dako Cytomation, Glostrup, Denmark; polyclonal 1:100). Sections were thawed for 30 min before rinsing for 15 min in PBS and 0.25% Triton X-100 at pH 7.2. Primary antibodies were diluted with PBS, 0.25% Triton X-100, and 1% bovine serum albumin (BSA) and incubated for 18–20 h at +4°C. After rinsing for 5 min × 3 in PBS and 0.25% Triton X-100, the sections were incubated with a secondary antibody (fluorescein isothiocyanate [Sigma] or Texas-Red [Jackson Immunoresearch, Suffolk, United Kingdom]) for 45 min in darkness in a 1:80 dilution with PBS, 0.25% Triton X-100, and 1% BSA. After another rinse in PBS and 0.25% Triton X-100 for 15 min × 2, they were mounted in antifading mounting medium.
Fluorescein-conjugated peanut agglutinin (PNA; Vector Laboratories, Burlingame, CA) was used to label interphotoreceptor matrix associated with cone photoreceptors. Retinal sections were thawed and rinsed in PBS with 1.0% BSA (PBS-BSA) for 30 min, after which they were incubated for 45 min with the fluorescein-conjugated PNA diluted 1:500 in PBS-BSA. After they were rinsed, PNA-labeled sections were mounted in Vectashield H-1000 (Vector Laboratories).
To identify dying (apoptotic) cells, a commercial terminal transferase-mediated dUTP nick-end labeling (TUNEL) assay system with fluorescein-conjugated dUTP was performed on the retinal sections according to the instructions of the manufacturer (Boehringer Mannheim, Mannheim, Germany).
For positive controls, immunohistochemical, PNA, and TUNEL labelings were performed on sections from normal adult rabbit eyes. Negative controls were obtained by performing the complete labeling procedure without the primary antibodies and fluorescein-conjugated PNA.
Sections were examined in a Nikon fluorescence microscope, and photographs were obtained with an attached Olympus digital camera system.
Results
Surgery and macroscopic examination
In all five rabbits, the approximately 45-μm-thick PGS membrane could be implanted into the subretinal space. One animal displayed a minimal choroidal hemorrhage (Fig. 2B), and another a limited sclerotomy-related hemorrhage at the end of surgery, but otherwise the procedures were uneventful.
Two days after surgery, all PGS membranes were identified in the subretinal space. In two eyes, a central retinal detachment was present, one eye had developed minimal cataract, and in one eye an inferior vitreous hemorrhage was present. At dissection 7 days after surgery (n = 2), the PGS membrane was found in place in the subretinal space. One eye displayed a retinal detachment surrounding the membrane, while in the other eye, the retina appeared attached on top of the membrane. After 28 days (n = 3), no PGS membrane was found, the retina was attached, and pigment alterations were seen in the area corresponding to the previous retinal detachment (Fig. 2C).
Morphology
Unoperated control eyes displayed a normal morphology on hematoxylin and eosin sections without any signs of inflammation or other pathology in the retina, RPE, or choroid. PNA labeling revealed well-labeled cone outer segments, and in most sections also the remaining parts of the cone photoreceptor. Sections labeled with the PKC antibody displayed normal rod bipolar cells, while GFAP-labeled sections displayed well-labeled astrocytes but no Müller cells.
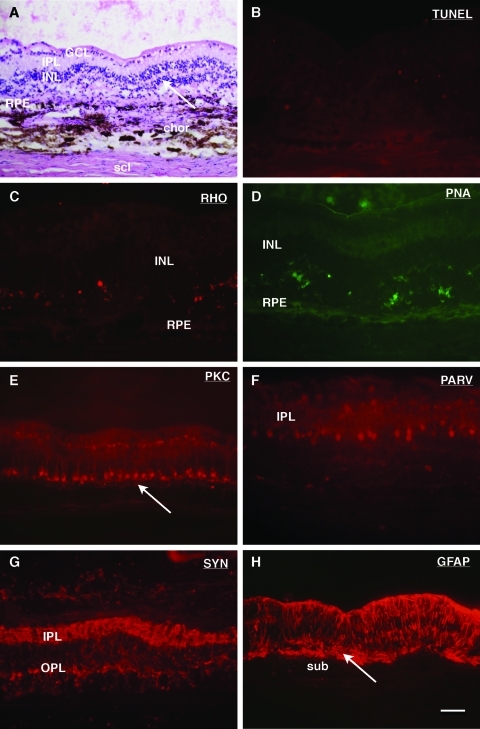
Hematoxylin and eosin–stained sections of operated eyes in 7-day specimens revealed a detached retina corresponding to the area of bleb formation, with the PGS membrane in place in the subretinal space. In one eye, a large gap in the RPE was present in the area adjacent to the membrane and the choroid displayed a multitude of inflammatory cells (Fig. 3A). In the remaining eye, the RPE monolayer was intact, but the individual cells had lost heir normal cuboid shape in favor of a more rounded or flat appearance. The outer nuclear layer (ONL) in the detached retina displayed fewer rows of cells compared with in areas of retinal attachment. TUNEL labeling revealed a multitude of apoptotic cells in the ONL within the implantation area (Fig. 3B). Within this layer, weakly labeled rhodopsin-positive rods as well as PNA-labeled cones were identified with short and disorganized inner segments, and no outer segments (Fig. 3C, D). PKC-labeled rod bipolar cells and parvalbumin-labeled AII amacrine cells displayed a normal arrangement and labeling pattern (Fig. 3E, F). Synaptophysin labeling revealed a normal thickness of the outer plexiform layers (OPL) and inner plexiform layers (IPL) (Fig. 3G). GFAP-labeled Müller cells were present in the entire retina, with no signs of subretinal gliosis in the detached area (Fig. 3H).
FIG. 3.
Histologic sections of the PGS implanted area 7 days postoperatively. (A) Hematoxylin and eosin staining. The membrane is seen in the subretinal space (black arrows), with an adjacent inflammatory mass in the choroid (chor). A neuroretinal detachment from the retinal pigment epithelium (RPE) is seen (white double-headed arrow). scl, sclera. (B) TUNEL labeling. A multitude of labeled cells can be seen in the outer nuclear layer (ONL) in the PGS-implanted area. (C) Rhodopsin (Rho) labeling. Labeling is present in the entire ONL with highest intensity in remaining inner segments (IS). (D) PNA labeling. Intense labeling is seen in inner segments of the cone photoreceptors (white arrows). (E) PKC labeling. Labeled rod bipolar cells display perikarya in the inner nuclear layer (INL) and extend axons terminating in the inner plexiform layer (IPL). (F) Parvalbumin (PARV) labeling. Well-labeled AII amacrine cells can be seen on the inner and outer side of the IPL. (G) Synaptophysin (SYN) labeling. Intense labeling is seen in the outer plexiform layer (OPL) as well as in the IPL. (H) GFAP labeling. Intense labeling of Müller cell fibers in the area of retinal detachment is seen. No Müller cell processes extending into the subretinal space (sub) are seen. Scale bars: (A, H) 100 μm; (B–G) 50 μm. Color images available online at www.liebertonline.com/tea
In specimens examined 28 days after PGS implantation, no retinal detachment could be seen in hematoxylin and eosin–stained sections (Fig. 4A). A substantial degeneration of the ONL, and disruption of the RPE were present, while inner retinal layers appeared intact. No inflammation and no sign of the PGS membrane were seen. A few scattered TUNEL-labeled cells could be seen in the implantation area (Fig. 4B). Rhodopsin-labeled rods and PNA-labeled cones were few and disorganized in this region (Fig. 4C, D), while PKC-labeled rod bipolar cells and parvalbumin-labeled AII amacrine cells displayed a normal arrangement and labeling pattern. The number of these cells in the previously detached area appeared as numerous as in the remaining retina, and they displayed a normal organization except for the dendritic processes of the rod bipolar cells that extended toward the subretinal space in a disorganized manner. Synaptophysin labeling revealed an IPL of normal thickness, whereas the OPL was found to be abnormally thin (Fig. 4G). GFAP-labeled Müller cells were seen throughout the retina, but the labeling intensity in areas outside the detachment area was lower compared to that in 7-day specimens. Within this area, labeling was strong, and subretinal ingrowth of Müller cell processes into the subretinal space was seen in some sections (Fig. 4H).
FIG. 4.
Histologic sections of the PGS-implanted area 28 days postoperatively. (A) Hematoxylin and eosin staining. The retina is attached to the disrupted retinal pigment epithelium (RPE). Only a few outer nuclear cells remain (arrow), whereas the inner nuclear layer (INL), inner plexiform layer (IPL), and ganglion cell layer (GCL) appear intact. scl, Sclera; chor, choroid. (B) TUNEL labeling. A few scattered labeled cells are present. (C) Rhodopsin (Rho) labeling. A few scattered positive cells can be seen in the outer part of the retina between the INL and the RPE. (D) PNA labeling. A few scattered labeled cells are present between the INL and the RPE. (E) PKC labeling. Well-organized rod bipolar cells are seen with weakly labeled disorganized dendrites extending toward the subretinal space (arrow). (F) Parvalbumin (PARV) labeling. Well-labeled AII amacrine cells can be seen on the inner and outer side of the IPL. (G) Synaptophysin (SYN) labeling. Intense labeling is seen in the IPL, which can be readily identified. The outer plexiform layer (OPL) appears thin and disorganized. (H) GFAP labeling. Intense labeling of Müller cell fibers in the area of retinal detachment is seen. Müller cell processes (arrow) extend into the subretinal space (sub). Scale bars: (A, H) 100 μm; (B–G) 50 μm. Color images available online at www.liebertonline.com/tea
Discussion
In this paper, we have shown that photoreceptors in the adult rabbit retina can be selectively removed by placing a membrane composed of the biodegradable elastomer (PGS) in the subretinal space. We closely followed our previously published protocol for transplanting full-thickness neuroretinal sheets,17 and found that the PGS membrane could be implanted with a minimal amount of complications. The membrane behaved similarly to neuroretinal tissue but was not as elastic and foldable, which in one eye resulted in RPE and choroidal damage. This eye displayed a localized inflammation at the implantation site 7 days after PGS implantation, most probably caused by a wound-healing response elicited by rupture of Bruch's membrane.18 No inflammation was found in the remaining eyes, which indicates that the PGS membrane was well tolerated in the subretinal space. The biodegradable nature of the membrane thus in most cases appears to allow gradual reattachment after detachment without concomitant inflammation.
The separation of neuroretina from the RPE has several consequences. In the normal retina, photoreceptor outer segments and microvilli of the RPE form an anatomic as well as a functional unit. A separation of the RPE from the outer segments leads to rapid pathological changes in both entities,19 which explains the early loss of outer segments as well as cellular changes in the RPE in eyes with subretinal PGS membranes. Extensive RPE changes are not desirable in a transplantation setting, and have fortunately not been seen in eyes transplanted with full-thickness neuroretina.20 Together, these results indicate that in future transplantation experiments, PGS and neuroretinal donor tissue should be implanted simultaneously, and not in succession.
Other effects of the subretinal PGS membrane stems from ischemia. The rabbit retina is merangiotic; that is, retinal blood vessels are confined to the myelinated streak, and the major part of the retina has been reported to be dependent on choroidal blood supply.21 The current experiment, as well as our previously published studies on full-thickness neuroretinal transplants, suggests that rabbit photoreceptors are more vulnerable to retinal detachment than inner retinal cells.17,20 The reduction of the ONL seen already after 7 days in combination with massive TUNEL labeling and almost complete ablation of this layer after 28 days suggests that the PGS membrane effectively blocks the diffusion of nutrients from the choroid leading to apoptotic cell death of photoreceptors. Inner retinal cells are evidently more resistant to this ischemic insult as found by the PKC and parvalbumin labeling, the normal appearance of the synaptophysin-labeled IPL, and the absence of TUNEL staining. This indicates that at least in the central part of the rabbit retina, inner retinal cells receive nutritive support from retinal vessels and/or from the vitreous. The fact that inner retinal neurons appear to survive the ischemic insult well in the merangiotic rabbit eye is encouraging, and logically this should also be the case in holangiotic animal models with a complete retinal circulation. However, the extent of photoreceptor degeneration in such a model may be different, and caution is therefore advocated when translating results to a holangiotic retina.
The inner retina is not completely unaffected in the model of choroidal ischemia. The rod bipolar cells in the implantation area displayed a disrupted dendritic network, which has been reported previously in experimental retinal detachment as well as in retinal degenerative disease.5,22 The phenomenon may be regarded as a part of a general retinal remodeling in which the eliciting event appears to be retraction of rod terminals. The fact that remodeling is also associated with ectopic synaptogenesis is of particular interest from a retinal transplantation point of view.23
Another effect of choroidal ischemia derived from the PGS implantation is GFAP upregulation in Müller cells, a sign of activation and glial remodeling known from vitrectomy procedures as well as from detachment.24,25 After 7 days, GFAP-labeled Müller cells were normally arranged, but after 28 days, some Müller cells in the implantation area had undergone hypertrophy with processes extending into the subretinal space. A gliotic reaction in the subretinal space is not advantageous for retinal transplantation and may warrant modifications of the PGS membrane in future experiments.
In the field of tissue engineering, degradable biomaterials are typically designed to foster new tissue generation with concomitant replacement of the biomaterial as the biomaterial degrades.26 In the present study, we have taken an antithetical approach by using a degradable biomaterial for the specific purpose of eliminating a tissue as part of a larger regeneration strategy. To our knowledge, the results reported here constitute the first example of using a biomaterial to eliminate a tissue as part of a general cell replacement therapy. A previous study on the biocompatibility of a nondegradable subretinal prosthetic reported unintended photoreceptor degeneration.27 Presumably, the prosthetic blocked the flow of nutrients from the choroid/RPE to the photoreceptor layer leading to the observed degeneration.
The PGS membrane used in this study was fabricated as a solid polymer sheet to eliminate or greatly diminish the flow of nutrients from the choroid to the photoreceptor layer. Since we previously developed a method for microfabricating a porous PGS membrane for subretinal implantation,10 future studies will seek to determine if the addition of low amounts of porosity to the PGS membrane can control the rate of photoreceptor degeneration and if changing the rate of degeneration provides any benefits for this procedure. As described above, subretinal gliosis is not desirable, and we also noted one instance of mechanical noncompliance during this study resulting in RPE and choroidal damage. We are currently investigating PGS membranes that are thinner and softer to address these issues. To reduce the number of surgical manipulations, and as mentioned above, minimize RPE trauma, it may prove possible to combine the PGS membrane with a healthy photoreceptor layer that could be implanted as a one step treatment for retinal degeneration. We are currently exploring methods for attaching a photoreceptor layer to PGS that would enable both components to be handled as a single entity.
To conclude, we have shown that photoreceptors in the rabbit retina can be selectively removed by retinal detachment induced by implantation of the biodegradable PGS membrane in the subretinal space. The stage is now set for transplantation experiments in which grafted photoreceptor cells, immature or adult, should have a reasonable potential of neuronal integration with a host retina devoid of photoreceptors but with remaining inner retinal cells. Since vertebrate retinal function is dependent on a precise organization of retinal neurons, we believe that the success of such a transplantation paradigm will depend greatly on the architecture of the graft. We have recently reported a technique of photoreceptor sheet isolation in vitro,28 and it will certainly be fascinating to explore transplantation of such grafts in combination with a PGS membrane.
Acknowledgments
This work was supported by The Faculty of Medicine, University of Lund, The Swedish Research Council, The Princess Margaretas Foundation for Blind Children, the Torsten and Ragnar Söderberg Foundation, the National Institutes of Health (Grants DE013023 and HL060435), and the Richard and Gail Siegal Gift Fund. W.L.N. was supported by the NIH under Ruth L. Kirschstein National Research Service Award 1 F32 EY018285-01 from the National Eye Institute.
Disclosure Statement
The authors confirm that no competing financial interests exist.
References
- 1.Seiler M.J. Aramant R.B. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest Ophthalmol Vis Sci. 1998;39:2121. [PubMed] [Google Scholar]
- 2.Ghosh F. Engelsberg K. English R.V. Petters R.M. Long-term neuroretinal full-thickness transplants in severely degenerated rhodopsin transgenic pigs. Graefes Arch Clin Exp Ophthalmol. 2007;245:835. doi: 10.1007/s00417-006-0437-9. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh F. Bruun A. Ehinger B. Graft-host connections in long-term full thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999;40:126. [PubMed] [Google Scholar]
- 4.Zhang Y. Arner K. Ehinger B. Perez M.T. Limitation of anatomical integration between subretinal transplants and the host retina. Invest Ophthalmol Vis Sci. 2003;44:324. doi: 10.1167/iovs.02-0132. [DOI] [PubMed] [Google Scholar]
- 5.Fisher S.K. Lewis G.P. Linberg K.A. Verardo M.R. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24:395. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y. Ameer G.A. Sheppard B.J. Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y. Kim Y.M. Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J Biomed Mater Res A. 2003;66:192. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 8.Gao J. Ensley A.E. Nerem R.M. Wang Y. Poly(glycerol sebacate) supports the proliferation and phenotypic protein expression of primary baboon vascular cells. J Biomed Mater Res A. 2007;83:1070. doi: 10.1002/jbm.a.31434. [DOI] [PubMed] [Google Scholar]
- 9.Sundback C.A. Shyu J.Y. Wang Y. Faquin W.C. Langer R.S. Vacanti J.P. Hadlock T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Neeley W.L. Redenti S. Klassen H. Tao S. Desai T. Young M.J. Langer R. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008;29:418. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J. Crapo P.M. Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 12.Bettinger C.J. Orrick B. Misra A. Langer R. Borenstein J.T. Microfabrication of poly (glycerol-sebacate) for contact guidance applications. Biomaterials. 2006;27:2558. doi: 10.1016/j.biomaterials.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Fidkowski C. Kaazempur-Mofrad M.R. Borenstein J. Vacanti J.P. Langer R. Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 14.Morra M. Occhiello E. Marola R. Garbassi F. Humphrey P. Johnson D. On the aging of oxygen plasma-treated polydimethylsiloxane surfaces. J Colloid Interface Sci. 1990;137:11. [Google Scholar]
- 15.Chaudhury M.K. Whitesides G.M. Correlation between surface free energy and surface constitution. Science. 1992;255:1230. doi: 10.1126/science.255.5049.1230. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhury M.K. Whitesides G.M. Direct measurement of interfacial interactions between semispherical lenses and flat sheets of poly(dimethylsiloxane) and their chemical derivatives. Langmuir. 1991;7:1013. [Google Scholar]
- 17.Ghosh F. Arnér K. Ehinger B. Transplant of full-thickness embryonic rabbit retina using pars plana vitrectomy. Retina. 1998;18:136. doi: 10.1097/00006982-199818020-00007. [DOI] [PubMed] [Google Scholar]
- 18.Kiilgaard J.F. Wiencke A.K. Scherfig E. Prause J.U. la Cour M. Transplantation of allogenic anterior lens capsule to the subretinal space in pigs. Acta Ophthalmol. 2002;80:76. doi: 10.1034/j.1600-0420.2002.800115.x. [DOI] [PubMed] [Google Scholar]
- 19.Anderson D.H. Stern W.H. Fisher S.K. Erickson P.A. Borgula G.A. Retinal detachment in the cat: the pigment epithelial-photoreceptor interface. Invest Ophthalmol Vis Sci. 1983;24:906. [PubMed] [Google Scholar]
- 20.Ghosh F. Johansson K. Ehinger B. Long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999;40:133. [PubMed] [Google Scholar]
- 21.de Schaepdrijver L. Simoens P. Lauwers H. de Geest J.P. Retinal vascular patterns in domestic animals. Res Vet Sci. 1989;47:34. [PubMed] [Google Scholar]
- 22.Marc R.E. Jones B.W. Watt C.B. Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y.W. Hao Y. Petters R.M. Wong F. Ectopic synaptogenesis in the mammalian retina caused by rod photoreceptor-specific mutations. Nat Neurosci. 2000;3:1121. doi: 10.1038/80639. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida A. Ishiguro S. Tamai M. Expression of glial fibrillary acidic protein in rabbit Müller cells after lensectomy-vitrectomy. Invest Ophthalmol Vis Sci. 1993;34:3154. [PubMed] [Google Scholar]
- 25.Erickson P.A. Fisher S.K. Guerin C.J. Anderson D.H. Kaska D.D. Glial fibrillary acidic protein increases in Muller cells after retinal detachment. Exp Eye Res. 1987;44:37. doi: 10.1016/s0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 26.Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory's experience. Acc Chem Res. 2000;33:94. doi: 10.1021/ar9800993. [DOI] [PubMed] [Google Scholar]
- 27.Pardue M.T. Ball S.L. Phillips M.J. Faulkner A.E. Walker T.A. Chow A.Y. Peachey N.S. Status of the feline retina 5 years after subretinal implantation. J Rehabil Res Dev. 2006;43:723. doi: 10.1682/jrrd.2005.07.0118. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh F. Arnér K. Engelsberg K. Isolation of photoreceptors in the cultured full-thickness fetal rat retina. Invest Ophthalmol Vis Sci. 2009;50:826. doi: 10.1167/iovs.08-2389. [DOI] [PubMed] [Google Scholar]