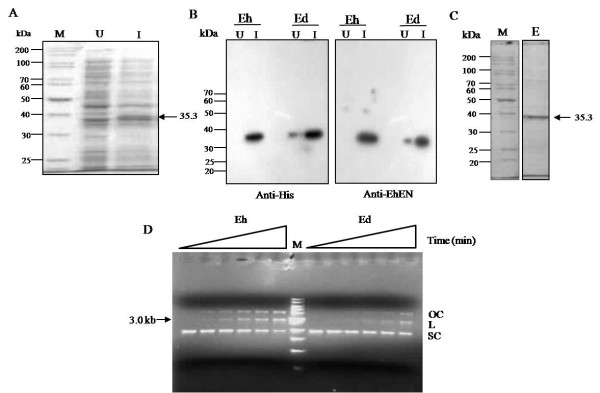
Figure 5.
Purification of Ed EN and cleavage activity on pBS supercoiled DNA. (A) The Ed EN protein was expressed in the E. coli expression vector pET30(b) by inducing with IPTG. Expression was checked by separating uninduced (U) and induced (I) whole cell lysate on 12% denaturing polyacrylamide gel. The expected size (35.3 kDa) is marked. (B) Western blot analysis with anti-his tag and anti-Eh EN antibody was performed using whole cell lysate from above figure. Eh EN was loaded for comparison in the 'Eh' lanes. (C) Ed EN was purified through Ni-NTA affinity chromatography and checked by separating on 12% denaturing polyacrylamide gel. (D) The cleavage activity of Ed EN was checked by incubating purified protein with pBS supercoiled DNA at 37°C under conditions earlier used for Eh EN. The figure shows agarose gel picture of time course (0, 5, 10, 15, 30 and 60 min) of incubation with Eh EN and Ed EN. The position of 3.0 kb band corresponding with linear pBS DNA is marked. OC, open circular; L, linear; SC, supercoiled.