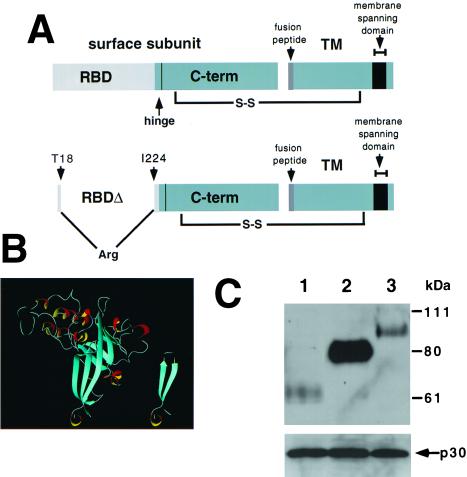
Figure 1.
(A) Schematic diagram of RBD in Fr-MLV envelope glycoprotein. The top diagram illustrates that RBD is composed of the N-terminal portion of the SU and is connected to the C-terminal portion of SU by a proline-rich “hinge.” The SU and TM are linked by a disulfide bond. The bottom diagram illustrates the location of the deletion (residues 19–223) and insertion of an arginine residue introduced to obtain Fr-MLV (env ΔRBD). (B) Proposed structure of Fr-MLV Env ΔRBD. Ribbon diagram of the RBD of the Fr-MLV surface glycoprotein (Left). It is composed of a barrel-like structure (blue) similar to an Ig-fold and a series of loops (white) and helices (red/yellow) at the top that contact receptor (10, 11). The N- and C-terminal β-strands are adjacent and together form the base of RBD. Env ΔRBD was prepared by deleting residues 19–223 and connecting these two β-strands by inserting an arginine residue between T18 and I224 at the top of the stalk. The env ΔRBD structure was modeled in the Swiss Protein Data Bank, selecting a structure that minimized steric clashes, and was visualized by using molmol (Right). (C) Incorporation of MLV envelope proteins into virions. Immunoblot of a nitrocellulose filter prepared from lysates of purified virions after SDS/PAGE. The top filter was probed with goat anti-MLV gp70 and HRP mouse anti-goat antibodies. Lane 1: lysate from Fr-MLV (env ΔRBD). Lane 2: lysate from wild-type Fr-MLV. Lane 3: lysate from Fr-MLV (Epo-env). To control for MLV particle production, aliquots of the same lysates were examined for the presence of the p30 capsid protein after SDS/PAGE by using goat anti-MLV and HRP mouse anti-goat antibodies (below).