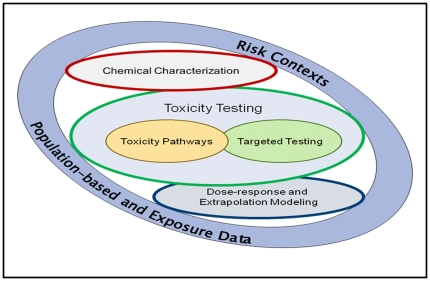
Figure 1. Schematic of components required to implement the new vision of toxicity testing in the 21st century (needs permission from NRC TT21C report).
The core components of the TT21C vision relate to testing for biological activity of compounds in toxicity pathway assays and to dose-response and extrapolation modeling using computational systems biology and pharmacokinetic tools. In the period of transition from current practice to the new vision some targeted testing in animals might be required. Targeted testing might also allow evaluation of target pathways and identification of metabolites. Assessing likely metabolism remains a challenge for the full implementation of the TT21C vision.