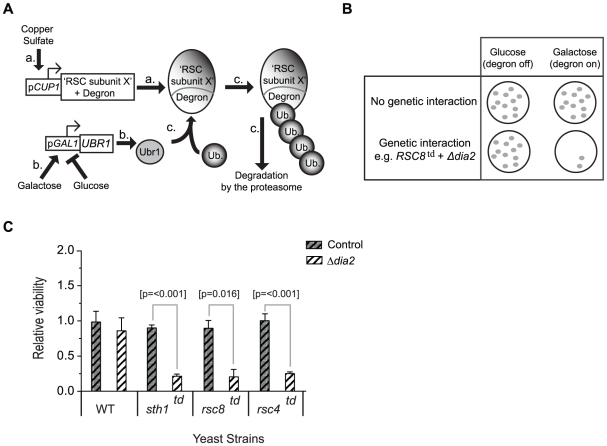
Figure 1. Dia2 genetically interacts with the RSC complex.
1A) Experimental design Each RSC degron strain harbours two inducible genes: a) One is a subunit of the RSC complex, fused to the N-end rule degron motif and driven by the CUP1 promoter. Addition of copper sulfate to the media induces expression of the degron construct (denoted by the td suffix). b) The second is the N-end rule ubiquitin ligase, Ubr1, driven by the GAL1 promoter. Addition of galactose causes Ubr1 expression and consequent ubiquitylation of the degron-tagged RSC subunit and causes its proteasome-dependent degradation. c) Degradation by this mechanism is optimal at 37°C and occurs at a reduced level at 30°C. Complete degradation of the RSC-degron subunits is not desired, as most RSC subunits are essential for cell survival. 1B) Genetic screen design Yeast strains harbouring degron-tagged RSC subunits were transformed with a dia2 knock-out cassette and grown in either dextrose (degron-repressing) or galactose (degron-activating) media. In parallel, a control set of transformations was conducted in the same way, using an irrelevant knock-out cassette (Δsuc2 control). If there was synthetic lethality between the RSC degron mutant and Δdia2, the number of viable colonies will be reduced in galactose but not in dextrose. 1C) Numerical analysis of the experiment described in 1B Synthetic lethality of Δdia2 was observed with the sth1td, rsc8td and rsc4td mutants. The interaction is specific as viability was only reduced when Δdia2 was transformed under degron-activating (‘Gal’/YPG) growth conditions. To normalise for the effects of carbon source on transformation efficiency, all colony counts were normalised against the value for the corresponding dextrose samples (relative viability = no. of colonies on YPG/ no. of colonies on YPD). Data presented represents three sets of technical replicates (n = 3).