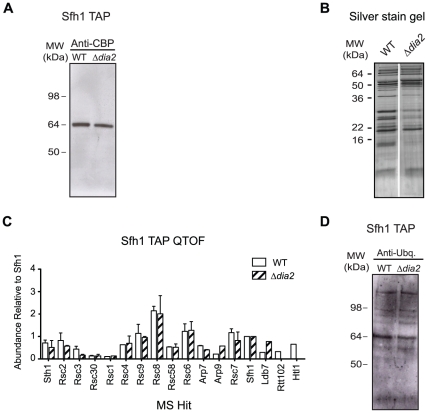
Figure 5. Dia2 is required for proper assembly of the RSC complex.
Tandem affinity purification (TAP) and subsequent analysis by tandem mass spectrometry was performed using an Sfh1-TAP expression strain in both the wild-type and Δdia2 backgrounds. 5A) Sfh1 pull-down of the RSC complex Western blotting shows efficient immunoprecipitation of TAP-tagged Sfh1 complexes from both the wild-type and Δdia2 strains. Sfh1 (recognised by the anti-calmodulin-binding protein antibody, anti-CBP) migrates at 64 kDa, as expected. 5B) RSC complex purified from the Δdia2 differed from the wild-type Silver stained gel showing proteins purified following TAP purification in the wild-type and Δdia2 strains (Δdia2 showed quantitative differences in immunoprecipitated proteins compared to wildtype). 5C) RSC complex in the Δdia2 mutant lacked subunits Semi-quantitative plot of differential abundance of protein complex components obtained by evaluating their exponentially modified protein abundance index (EmPAI) following analysis with tandem mass spectrometry. Error bars represent standard error of the mean from two biological replicates. 5D) Pattern of ubiquitylation did not differ between wild-type and Δdia2 RSC complexes Western blotting of Sfh1 immunopurified RSC complexes using an anti-ubiquitin antibody. Multiple bands potentially representing ubiquitylated subunits of the RSC complex are present equally in wild-type and Δdia2 samples.