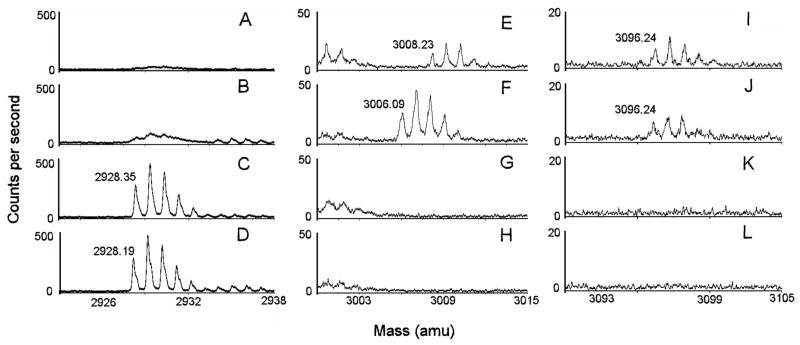
Fig 2.
MALDI TOF mass spectra of the active site tryptic peptide from reactions of BChE with CBDP in the presence and absence of 18O-water.
Reactions of BChE with CBDP were conducted as described in the Materials and Methods section. Masses of interest are shown: 2928 amu [M+H]+1 for the unlabeled active site peptide SVTLFGESAGAASVSLHLLSPGSHSLFTR in positive mode; 3006 amu [M-H]−1 for the phosphorylated active site peptide in the presence of 16O-water in negative mode; 3008 amu [M-H]−1 for the phosphorylated active site peptide in the presence of 18O-water in negative mode; 3096 amu [M-H]−1 for the o-cresyl-phosphorylated active site peptide in the presence of 18O-water and 16O-water in negative mode. Limited mass ranges around the masses of interest are shown to emphasize the isotopic splittings and peak resolution, to illustrate the relative intensities of the members of the isotopic families, and to avoid interference from other peptides with greater signal intensities. Panels A, E and I are from the reaction of BChE with CBDP in the presence of 18O-water; panels B, F and J are from the reaction of BChE with CBDP in the presence of 16O-water; panels C, G and K are from an incubation of BChE in 18O-medium without CBDP; and panels D, H and L are from an incubation of BChE in 16O-medium without CBDP. A mass shift of +2 from incorporation of 18O-water is seen only in panel E for the phosphorylated active site peptide of mass 3008, which demonstrates that the P-O bond was broken when the cresylphosphate adduct aged to form phospho-serine. In contrast 18O was not introduced during formation of the cresylphosphate adduct (mass 3096 in panels I and J), which means this adduct resulted from cleavage of the O-C bond.