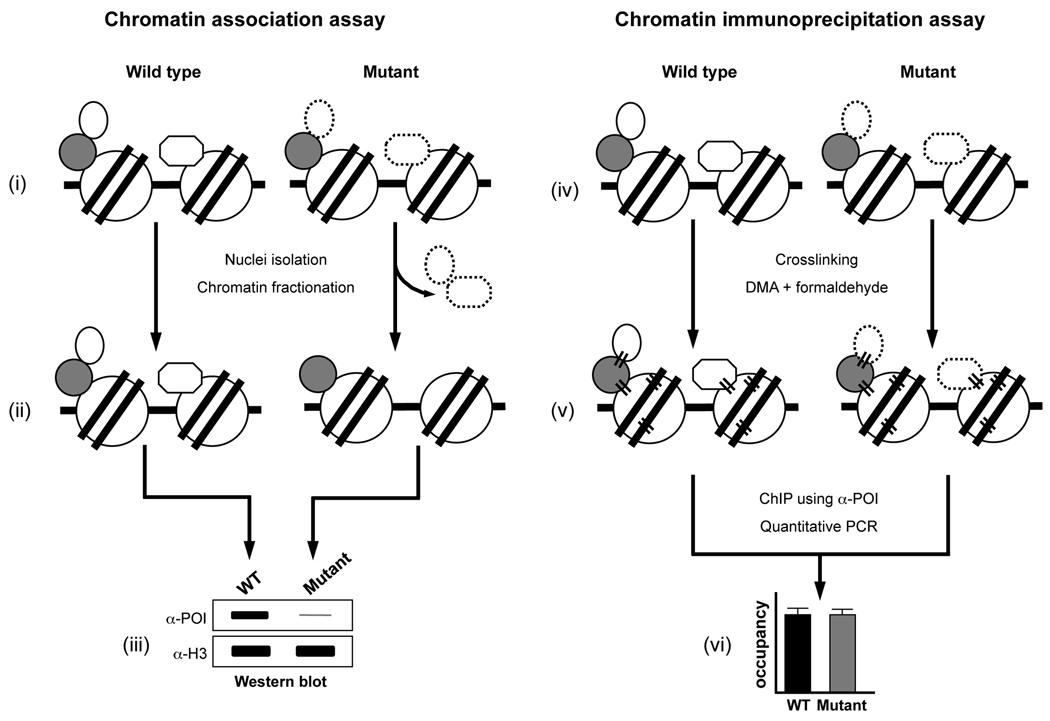
Fig. 5.
A combinatorial approach employing chromatin association and ChIP assays can reveal defects in protein-protein interactions on chromatin. Schematic representations of the chromatin association assay and ChIP assay are shown. A speculative scenario of destabilization in the interactions of a chromatin-bound protein under certain experimental condition (mutation or treatment) compared to the control (wild type or untreated) and discrepancy/difference in the detection of this destabilized interaction between the two assays are depicted. In (i) and (iv), a protein of interest (POI, oval or octagon) is shown stably associated with the wild type chromatin either by its interactions with a partner or bridging protein (gray circle) or via its direct interaction with the nucleosome (histone octamer, circle; DNA, solid line). These direct and indirect interactions of the POI may be destabilized or weakened in the mutant without any reduction in its overall levels on chromatin (oval or octagon with dashed line). (ii) The destabilized/weakened interactions might render the POI susceptible to dissociation from the chromatin during the multiple steps, with solutions of different ionic strengths, involved in the chromatin association assay (nuclei isolation and chromatin fractionation) and resulting in the reduced levels of POI on the chromatin in the mutant. (iii) Normalized soluble chromatin is subjected to Western blotting and an antibody (α-POI) is used to measure changes in the chromatin-bound levels of POI in the wild type or mutant. The amount of H3 is used to confirm that equal amounts of chromatin were used in the assay (detected using α-H3, loading control). As shown in the mock-up Western blot, chromatin-bound levels of POI in the mutant might be highly reduced (thin gray line) due to its dissociation as compared to the wild type. (v) Addition of a bifunctional crosslinking agent (DMA) and formaldehyde causes protein-protein and protein-DNA crosslinking (parallel black lines) and results in stable tethering of the POI to chromatin. Crosslinking stabilizes the “loose” or weakened interactions of POI in the mutant and prevents its dissociation during the several incubation and wash steps (including highly stringent conditions, such as the exposure to 500 mM NaCl and detergents) involved in the ChIP assay. (vi) Soluble chromatin is prepared from the wild type and mutant strains subjected to double crosslinking and IP is performed using α-POI. Changes in the chromatin-bound levels of POI (occupancy) in the wild type and mutant are determined using qPCR analysis of input and ChIP DNA. As depicted in the model graph, owing to crosslinking, occupancy of the POI on chromatin in the mutant might be similar to that in the wild type, unlike in chromatin association assay (iii).