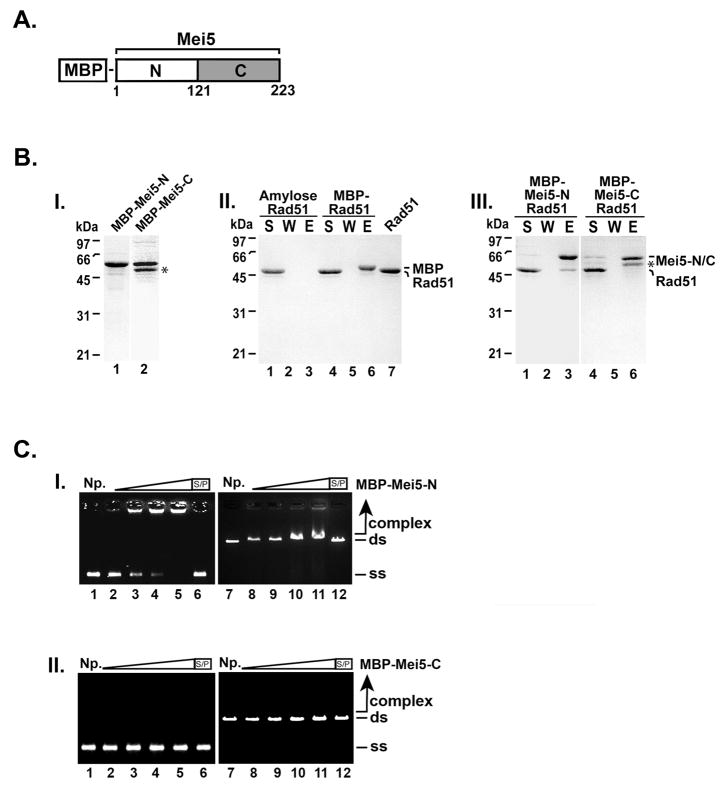
Figure 5. The N-terminal domain of Mei5 interacts with Rad51 and binds DNA.
(A) Domain schematic of Mei5. (B) MBP-Mei5-N (1.3 μg; panel I, lane 1) and MBP-Mei5-C (1.2 μg; panel I, lane 2) were resolved using a 12% SDS-PAGE polyacrylamide gel and stained with Coomassie Blue. Rad51 (7 μg) was incubated with MBP (6.5 μg; panel II, lanes 4–6), MBP-Mei5-N (7.0 μg; panel III, lanes 1–3), MBP-Mei5-C (7.0 μg; panel III, lanes 4–6) for 30 min at 4°C. Amylose beads were added to the reactions and with Rad51 alone (panel II, lanes 1–3) for 30 min at 4°C with agitation to capture protein complexes. The supernatant (S) that contained unbound proteins, wash (W), and SDS eluate (E) were analyzed by SDS-PAGE and stained with Coomassie Blue. (C) MBP-Mei5-N and MBP-Mei5-C (panel I and panel II, respectively; 0.55 μM, lanes 2 and 8; 1.66 μM, lanes 3 and 9; 2.77 μM, lanes 4 and 10; 5.5 μM, lanes 5, 6, 11 and 12) were incubated with φX174 ssDNA (30 μM nucleotides, lanes 2–6) and linearized φX174 RF dsDNA (30 μM base pairs, lanes 7–12) at 37°C for 10 min. The reaction products were separated on 0.9 % agarose gels and stained with ethidium bromide. Where indicated, the reaction was treated with SDS (0.5% final) and Proteinase K (0.5 mg/mL) at 37°C for 15 min prior to analysis. S/P, SDS/Proteinase K; NP, no protein control; ss, ssDNA; ds, dsDNA. (*) Indicates the C-terminal truncation product of MBP-Mei5.