Abstract
Epoxyeicosatrienoic acids (EETs) are vasodilator, natriuretic, and antiinflammatory lipid mediators. Both cis- and trans-EETs are stored in phospholipids and in red blood cells (RBCs) in the circulation; the maximal velocity (Vmax) of trans-EET hydrolysis by soluble epoxide hydrolase (sEH) is threefold that of cis-EETs. Because RBCs of the spontaneously hypertensive rat (SHR) exhibit increased sEH activity, a deficiency of trans-EETs in the SHR was hypothesized to increase blood pressure (BP). This prediction was fulfilled, since sEH inhibition with cis-4-[4-(3-adamantan-1-ylureido)cyclohexyloxy]benzoic acid (AUCB; 2 mg·kg−1·day−1 for 7 days) in the SHR reduced mean BP from 176 ± 8 to 153 ± 5 mmHg (P < 0.05), whereas BP in the control Wistar-Kyoto rat (WKY) was unaffected. Plasma levels of EETs in the SHR were lower than in the age-matched control WKY (16.4 ± 1.6 vs. 26.1 ± 1.8 ng/ml; P < 0.05). The decrease in BP in the SHR treated with AUCB was associated with an increase in plasma EETs, which was mostly accounted for by increasing trans-EET from 4.1 ± 0.2 to 7.9 ± 1.5 ng/ml (P < 0.05). Consistent with the effect of increased plasma trans-EETs and reduced BP in the SHR, the 14,15-trans-EET was more potent (ED50 10−10 M; maximum dilation 59 ± 15 μm) than the cis-isomer (ED50 10−9 M; maximum dilation 30 ± 11 μm) in relaxing rat preconstricted arcuate arteries. The 11,12-EET cis- and trans-isomers were equipotent dilators as were the 8,9-EET isomers. In summary, inhibition of sEH resulted in a twofold increase in plasma trans-EETs and reduced mean BP in the SHR. The greater vasodilator potency of trans- vs. cis-EETs may contribute to the antihypertensive effects of sEH inhibitors.
Keywords: epoxyeicosatrienoic acids, soluble epoxide hydrolase, hypertension, cytochrome P-450
epoxyeicosatrienoic acids (EETs) are vasodilator, natriuretic, and anti-inflammatory lipid mediators (5, 6, 16, 30, 53). In the circulation, EETs are primarily esterified in plasma phospholipids and stored in red blood cell (RBC) membranes (22, 27, 41). While lipid peroxidation can produce cis- and trans-EETs (26, 27), this is unlikely a direct source because of the presence of multiple antioxidant systems in vivo (19, 47). NADPH-dependent cytochrome P-450 (CYP) metabolism of arachidonic acid produces only cis-EETs (8, 50, 56), whereas hydroperoxide-dependent CYP cooxidation of arachidonic acid can result in formation of both cis- and trans-EETs (4, 7, 52). Indeed, we have found CYP isoforms stimulated by lipid hydroperoxides preferentially produce trans- over cis-EETs (Jiang, unpublished observations), and we were the first to report the presence of trans-EETs in vivo (26, 27). Isomers of cis- and trans-EETs possess an equipotent platelet antiaggregatory activity (17, 26), but both 5,6-trans-EET and its hydration product 5,6-erythro-dihydroxyeicosatrienoic acids (DHET) exhibited greater vasodilator effects than 5,6-cis-EET (26).
EETs are antihypertensive, since increased endothelial epoxygenase expression significantly reduced blood pressure (BP) in hypertensive mice (32), and intra-arterial or intravenous infusion of 14,15-EET reduced BP in the spontaneously hypertensive rat (SHR) by a maximum of 45 mmHg (36). Moreover, inhibition of EET production by clotrimazole increased BP by 23 mmHg in Dahl rats fed a high-salt diet (38). EETs are hydrolyzed by soluble epoxide hydrolase (sEH) to DHETs, which generally have less biological activity than EETs. Increased sEH activity has been associated with hypertension (1, 18, 24, 55). Inhibition of EET hydrolysis by sEH inhibitors reduced BP by 20 to 30 mmHg in rats with angiotensin II (ANG II)-/salt-induced hypertension (21, 24, 37), as well as in the SHR (55) and the stroke-prone SHR (34). Deletion of the sEH gene reduced BP by 16 mmHg compared with wild-type DOCA-salt hypertensive mice (39). Inhibition of sEH is a potential therapeutic approach for control of BP, vascular inflammation, and for providing cardiac and renal protection by raising levels of EETs in vivo (45, 49).
RBCs represent a major source of sEH in the circulation for the hydration of cis- and trans-EETs, producing threo- and erythro-DHETs, respectively (29). The maximal velocity (Vmax) for the hydrolysis of trans-EETs is approximately three times greater than for their respective cis-isomers (29). The 14,15-trans-EET is hydrolyzed the fastest by rat RBCs among all EET isomers, and the rate of EET hydrolysis decreased two- to threefold sequentially from 14,15-, 11,12-, and 8,9- to 5,6-EETs (29). Inhibition of sEH will favor the preservation of trans-EETs over cis-EETs, since trans-EETs are hydrolyzed three times more rapidly than cis-EETs by sEH, and the Michaelis constant for trans- vs. cis-EETs is on average 1.6 vs. 1.0 μM, respectively (29). There is greater sEH expression and activity in SHR than in the normotensive Wistar-Kyoto rat (WKY) control strain (18, 48, 55).
RBCs are also reservoirs and carriers of EETs in the circulation (22, 27). RBCs, in addition to O2 delivery in the microvasculature, participate in the regulation of microvascular tone by releasing vasoactive factors, including EETs (25, 28). The role of RBC sEH in the regulation of circulating EETs may be particularly significant when considering that there are effects of EETs on the rheological and hemodynamic determinants of the circulation. Inhibition of RBC sEH presumably contributes to elevating effects of EETs on regional blood flows to a greater degree than inhibition of sEH localized “in the smooth muscle layers of the arterial wall” (54). The present studies focused on effects of the potent sEH inhibitor, cis-4-[4-(3-adamantan-1-ylureido)cyclohexyloxy]benzoic acid (AUCB) (23, 29), on BP and EET levels in the circulation in relation to the activity of RBC sEH in the SHR compared with the WKY, as well as the direct vasorelaxant activity of cis- vs. trans-EETs in renal microvessels.
MATERIALS AND METHODS
Animals.
The experimental procedures in animal use were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. Ten-week-old male SHR and WKY rats were purchased from Taconic Farms. Seven-week-old male Sprague-Dawley (SD) rats were from Charles River Laboratories. Rats were maintained at 22°C with alternating cycles of light and darkness and fed ad libitum with standard rat chow (Purina) and water. AUCB (0.01 mg/ml to provide a dose of 2 mg·kg−1·day−1) was dissolved in drinking water containing 0.2% ethanol and administered to 11-wk-old SHR and WKY rats for 7 days while the vehicle control rats were given tap water containing 0.2% ethanol.
BP measurements.
Noninvasive BP measurements were made using a volume-pressure tail-cuff system (Kent Scientific) (15). Each rat was subjected to five acclimation cycles followed by five measurement cycles for BP readings. In some cases, BP was measured by radiotelemetry (35) to confirm the changes measured by tail cuff.
Reagents.
Standard racemic trans-EETs were synthesized as described, and the purity as analyzed by HPLC was over 99% (14, 26). Standard cis-EETs were from Cayman Chemical (Ann Arbor, MI). AUCB was provided by Dr. Hammock and has been characterized as a potent sEH inhibitor (23, 29). BSA and HPLC-grade organic solvents were obtained from Fisher Scientific (Pittsburgh, PA), and EET-d8 standards were from Enzo Life Sciences (Plymouth Meeting, PA). Other reagents were purchased from Sigma (St. Louis, MO).
RBC preparation and incubation.
Preparation and incubation of rat RBCs was performed as previously described (29). Briefly, up to 10 ml blood were drawn from the inferior vena cava of pentobarbital-anesthetized rats. RBCs were isolated by centrifugation at 400–800 g and then washed with cold physiological salt solution after the buffy layer was discarded. RBC incubations to compare the hydrolysis of EETs by rat RBCs were carried out using 16 ng of cis- or trans-EETs in 50 μl prewarmed 5 × 108 RBCs in PBS at 37°C for 10 min with shaking at 600 rpm in a VWR 3-mm orbital shaker.
Plasma and urine EET extraction.
Rat plasma was obtained after centrifuging blood at 2,000 g for 10 min and mixed with polymer-bound triphenylphosphine (TPP, 1 mg/ml) to quench free radical-induced lipid peroxidation. Phospholipid was extracted from 0.4 ml plasma using the Bligh-Dyer (3) method and hydrolyzed with 1 M NaOH for 90 min at room temperature. The hydrolysis mixture was then neutralized with 1 M HCl and extracted two times with 2 ml ethyl acetate. The ethyl acetate extract was dried under a gentle stream of nitrogen and dissolved in acetonitrile (20 μl) for immediate LC/MS/MS analysis (27).
Rat urines (24 h) were collected in tubes containing 5 mg polymer-bound TPP. Urine samples (2 ml) with added d11-labeled 8,9- and 14,15-DHET and d8-labeled 8,9-, 11,12-, and 14,15-EET (1 ng each) were vigorously mixed two times with 3 ml hexane-ethyl acetate (1:1) to extract EETs and DHETs. The combined organic phase was backwashed with 4 ml of water, dried under a gentle stream of N2, and dissolved in 80 μl acetonitrile for HPLC separation and GC/MS analysis as described (26, 41).
Mass spectrometry analyses.
ESI LC/MS/MS analyses of EETs and DHETs were carried out as described (27, 29). Briefly, a Finnigan LCQ Advantage quadrupole ion-trap mass spectrometer equipped with ESI source run by XCALIBUR software was used. MS/MS breakdown for mass-to-charge ratio (m/z) 319 was at an energy level of 35% set by the instrument, and a seven-point Gaussian smoothing was applied in the mass data processing. Quantification of individual EETs was based on standard curves (r = 0.99) between their respective characteristic fragmentation ions with reference to an internal standard of 2 ng of d8–11,12-EET.
For quantification using electron-capture negative-chemical ionization GC/MS, purified DHET samples were derivatized to trimethylsilyl ether pentafluorobenzyl (PFB) esters, and EETs were derivatized to PFB esters as described (26, 28). The ions m/z of 481 and 492 were monitored for endogenous and d11-labeled DHETs; the ions m/z of 319 and 327 were monitored for endogenous and d8-labeled EETs.
Western blot analysis of sEH.
RBC cytosol was obtained by centrifugation of lysed RBCs at 10,000 g for 1 h and then diluted 1:2 with 10 mM Tris·HCl (pH 7.5), 1 mM EDTA, and 1% SDS on ice. Total protein concentration was quantified with the Pierce BSA assay using Fraction V BSA as the calibrating standard. For each sample, 50 μg of protein were loaded on a 12% SDS-PAGE, and Western blot analysis was carried out as described (29). Expression of GAPDH was detected using a monoclonal mouse antibody and a goat anti-mouse IgG labeled with horseradish peroxidase. Bands were visualized using the ECL kit from Amersham and results calculated as a ratio relative to GAPDH expression.
Rat renal arcuate artery studies.
Activities of cis- and trans-EET regioisomers were evaluated in microdissected SD rat arcuate arteries (∼100 μm internal diameter) that were cannulated and pressurized to 80 mmHg as described (12). Arcuate arteries were chosen because of their exquisite sensitivity to cis-EETs (12). We determined that neither indomethacin (10 μM), a cyclooxygenase inhibitor, nor l-NAME (200 μM), a nitric oxide synthase inhibitor, affected the vasoactivity of cis- and trans-EETs (data not shown). The vessels were preconstricted with phenylephrine (10−7 mol/l), and cumulative concentration responses to individual EET regioisomers were recorded before and after application of 14,15-EEZE (10−6 mol/l), a selective EET antagonist (20). We also examined the activity of the 14,15-EET isomers on arcuate arteries obtained from 12-wk-old SHR and WKY rats. In these experiments, arteries were pressurized to 80 and 120 mmHg to address any effects of different baseline pressures that exist in SHR and WKY rats.
Data analysis.
Results are presented as means ± SE and analyzed using GraphPad Prism 5 (San Diego, CA) software. Comparisons among multiple groups were made by ANOVA followed by Tukey's test for differences between groups. Paired and unpaired t-tests were used for comparison between two groups where appropriate. A P value <0.05 was considered as statistically significant.
RESULTS
Increased RBC sEH activity and expression in SHR compared with WKY.
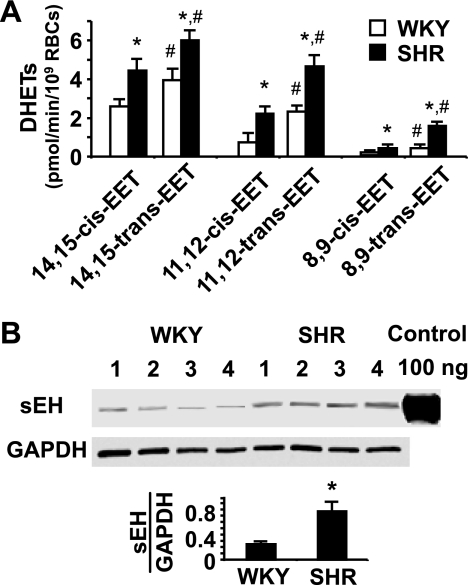
We examined RBC sEH activity and expression in SHR and WKY rats, since elevated sEH expression has been reported to occur in both the kidney and the brain of SHR (18, 48, 55). Incubation of cis- and trans-isomers of 14,15-, 11,12-, and 8,9-EETs (1 μM each) with RBCs from the 11-wk-old SHR and WKY revealed significant differences in the hydrolysis of EETs. Specific rates of EET hydrolysis by RBCs of the SHR are on average more than double that of the WKY (Fig. 1A). Greater hydrolysis of trans- than cis-EETs by RBCs from both the WKY and the SHR was also found (Fig. 1A), which is consistent with a Vmax for the hydrolysis of trans-EETs that is three times greater than that of cis-EETs (29).
Fig. 1.
Increased soluble epoxide hydrolase (sEH) activity and expression in red blood cells (RBCs) of the spontaneously hypertensive (SHR) vs. the Wistar-Kyoto (WKY) rat. A: hydrolysis of epoxyeicosatrienoic acids (EETs) by RBCs from the SHR and the WKY at 37°C for 10 min. DHET, dihydroxyeicosatrienoic acid. *P < 0.05 compared with RBCs from the WKY for hydrolysis of the same EET isomer, n = 6. #P < 0.05 compared with hydrolysis of the cis-isomer, respectively, n = 6. B: Western blot analysis of sEH in the RBC cytosol from the WKY and the SHR. *P < 0.05 compared with the WKY, n = 4.
The greater RBC sEH activity in SHR compared with WKY is consistent with the increased expression of sEH in the RBC cytosol of the SHR compared with the WKY (Fig. 1B). These results support previous reports indicating increased sEH expression and activity in hypertension (1, 24, 48, 55). Increased sEH activity has been postulated to be responsible for reduced plasma EET levels in essential hypertension (41).
Changes in plasma and urinary EETs and DHETs with sEH inhibition.
In 12-wk-old SHR treated for a week with AUCB, total plasma EETs in the SHR increased 22%, mostly reflecting an increase in plasma trans-EETs (Table 1). Plasma total EETs in the WKY remained unchanged with AUCB treatment (26.1 ± 1.8 vs. 28.5 ± 3.2 ng/ml). Total plasma EETs were higher in untreated WKY than SHR rats (26.1 vs. 16.4 ng/ml), but a significant elevation in response to sEH inhibition was only seen in the SHR (Table 1).
Table 1.
Plasma concentrations of individual cis- and trans-EETs in WKY and SHR treated with vehicle or AUCB for 1 wk
| WKY |
SHR |
|||
|---|---|---|---|---|
| Vehicle | AUCB | Vehicle | AUCB | |
| 14,15- | ||||
| cis-EET | 5.8 ± 0.6 | 5.2 ± 0.7 | 4.5 ± 0.6 | 4.4 ± 0.7 |
| trans-EET | 1.6 ± 0.2 | 3.4 ± 0.6* | 1.1 ± 0.1 | 2.3 ± 0.7* |
| 11,12- | ||||
| cis-EET | 2.1 ± 0.3 | 2.7 ± 0.3 | 1.4 ± 0.3 | 1.6 ± 0.2 |
| trans-EET | 2.0 ± 0.2 | 3.6 ± 0.8* | 0.9 ± 0.1 | 2.0 ± 0.5* |
| 8,9- | ||||
| cis-EET | 4.9 ± 0.4 | 3.3 ± 0.4 | 3.2 ± 0.4 | 3.0 ± 0.3 |
| trans-EET | 1.8 ± 0.3 | 2.7 ± 0.7* | 0.9 ± 0.1 | 1.9 ± 0.3* |
| 5,6- | ||||
| cis-EET | 5.5 ± 0.4 | 4.8 ± 0.3 | 3.2 ± 0.4 | 3.1 ± 0.2 |
| trans-EET | 2.4 ± 0.1 | 2.8 ± 0.5 | 1.2 ± 0.1 | 1.7 ± 0.3 |
| Total | ||||
| cis-EET | 18.3 ± 1.4 | 16.0 ± 1.5 | 12.3 ± 1.6 | 12.1 ± 1.3 |
| trans-EET | 7.8 ± 0.5 | 12.5 ± 1.9* | 4.1 ± 0.2 | 7.9 ± 1.5* |
| Overall | ||||
| EETs | 26.1 ± 1.8 | 28.5 ± 3.2 | 16.4 ± 1.6 | 20.0 ± 2.4* |
Values were determined by LC/MS/MS and expressed as means ± SE (ng/ml); n = 6–8 rats in each group. EET, epoxyeicosatrienoic acid; AUCB, cis-4-[4-(3-adamantan-1-ylureido)cyclohexyloxy]benzoic acid. Eleven-week-old Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats were treated with vehicle or AUCB (2 mg · kg−1 · day−1 in the drinking water) for 7 days.
P < 0.05 compared with plasma concentrations of vehicle rats of the same strain.
Total plasma cis-EETs were twofold greater than trans-EETs in control WKY rats (18.3 vs. 7.8 ng/ml). AUCB treatment did not affect plasma total cis-EET concentrations in the WKY and the SHR (Table 1). However, AUCB increased plasma trans-EET levels similarly in both the SHR (4.1–7.9 ng/ml) and WKY (7.8–12.5 ng/ml), viz., ∼4 ng/ml, which had the greater impact on plasma trans-EETs in the AUCB-treated SHR, given the relatively low total plasma EETs of the SHR (Table 1). Except for 5,6-trans-EET, a poor substrate for the sEH (10, 29), other trans-EETs were increased significantly in both strains when treated with the sEH inhibitor (Table 1).
Rat plasma total DHETs were ∼10% of the level of total EETs. A decrease in plasma DHETs was not found as expected with sEH inhibition (vehicle vs. AUCB-treated rats: 2.4 ± 0.6 vs. 2.7 ± 0.6 ng/ml for WKY; 1.7 ± 0.2 vs. 2.0 ± 0.3 ng/ml for SHR), possibly reflecting a dynamic balance among formation, metabolism, and excretion (51).
Total EETs and DHETs in rat urine were analyzed by GC/MS. Rat urinary excretion was mostly in the form of DHETs, whereas EETs accounted for <20% of the total. AUCB treatment for 7 days did not affect the urinary excretion of EETs or DHETs in either SHR or WKY rats. However, the total urinary excretion of EETs and DHETs was ∼50% less in SHR than in WKY on all days measured (for example, on day 7, DHET excretion with vehicle vs. AUCB was 42 ± 4 vs. 40 ± 5 for WKY and 27 ± 4 vs. 24 ± 3 ng/day for SHR, n = 8).
AUCB administration inhibits RBC hydrolysis of EETs.
To examine the effect of AUCB treatment on sEH activity, RBCs from the control and treated WKY and SHR were separated and tested for the hydrolysis of 1 μM 14,15-cis- and 14,15-trans-EET. RBCs from AUCB-treated rats exhibited a greatly reduced capacity to hydrolyze cis- and trans-EETs; hydrolysis of 14,15-trans-EET by RBCs of the AUCB-treated SHR was inhibited by 60.0 ± 3.2% (n = 8), whereas that of 14,15-cis-EET was inhibited by 41.1 ± 1.9% (n = 6) compared with RBCs from the vehicle SHR; similar percent inhibition was also found in the WKY (Table 2), consistent with the greater increase in trans-EETs compared with cis-EETs on sEH inhibition.
Table 2.
Conversion of 14,15-cis-EET to 14,15-threo-DHET and 14,15-trans-EET to 14,15-erythro-DHET by RBCs of vehicle- and AUCB-treated WKY and SHR
| DHET Produced, pmol · min−1 · 10−9 RBCs |
||
|---|---|---|
| Rat RBCs | 14,15-threo- | 14,15-erythro- |
| WKY vehicle | 2.82 ± 0.34 | 4.37 ± 0.52# |
| WKY AUCB | 1.67 ± 0.20* | 1.84 ± 0.22* |
| SHR vehicle | 4.40 ± 0.53 | 7.02 ± 0.70# |
| SHR AUCB | 2.59 ± 0.31* | 2.81 ± 0.34* |
Values are means ± SE; , n = 6-8 rats in each group. DHET, dihydroxyeicosatrienoic acid; RBC, red blood cell. 14,15-cis- and 14,15-trans-EET (1 μM each) were added to RBCs (5 × 108 RBCs in 50 μl PBS) of the vehicle control and the AUCB-treated SHR and WKY for 10 min at 37°C.
P < 0.05 compared with vehicle control rat RBCs for hydrolysis of the same 14,15-EET isomer. #P < 0.05 compared with the hydrolysis of 14,15-cis-EET by the same RBCs.
RBCs from the vehicle-treated SHR showed the greatest capacity to generate both erythro- and threo-DHETs (7.02 ± 0.70 and 4.40 ± 0.53 pmol·min−1·10−9 RBCs, respectively), in agreement with elevated expression of sEH in the SHR (Fig. 1B). Treatment of the SHR with AUCB reduced its RBC hydrolysis of trans-EETs, resulting in equalization of erythro- and threo-DHET production, 2.81 vs. 2.59 pmol·min−1·10−9 RBCs, respectively (Table 2). The lesser production of DHETs by vehicle-treated WKY RBCs compared with that of the vehicle-treated SHR once again reflects differences in RBC sEH activity in the two strains. As was the case for SHR, sEH inhibition in WKY resulted in equalization of erythro- and threo-DHET production, viz., 1.84 vs. 1.67 pmol·min−1·10−9 RBCs, respectively (Table 2).
BP reduction in the SHR is associated with increases in plasma trans-EETs.
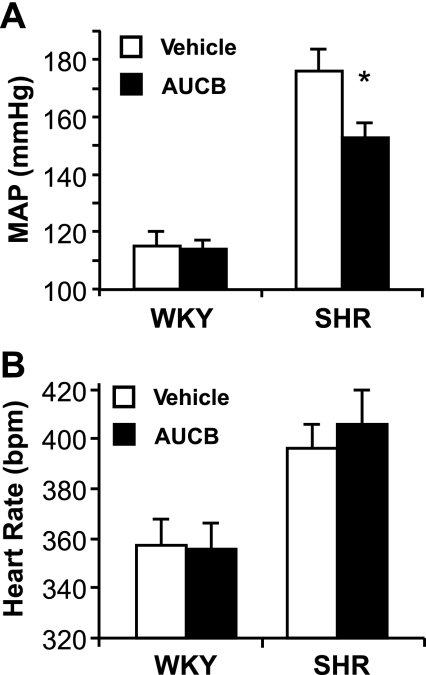
Mean arterial BP fell from 176 ± 8 to 153 ± 5 mmHg in the SHR, whereas it was unaffected in the WKY after 1 wk of treatment with AUCB (Fig. 2A). The reduction in BP with AUCB treatment in SHR reached a maximum in a week although BP was significantly reduced by day 3. Continued AUCB treatment did not result in a further decrease in BP. We anticipate that the effect of AUCB was maximal based on other studies of the potency of AUCB in inhibiting sEH (23, 29). The heart rate was not changed with AUCB treatment in either WKY rats or SHR (Fig. 2B).
Fig. 2.
Effects of 1 wk oral administration of cis-4-[4-(3-adamantan-1-ylureido)cyclohexyloxy]benzoic acid (AUCB) on mean arterial blood pressure (MAP, A) and heart rate (B). *P < 0.05 compared with vehicle-treated WKY or SHR of the same strain, n = 6–8.
The BP reduction is consistent with significant increases in plasma trans-EET concentrations in the SHR (Table 1). Although inhibition of sEH also increased trans-EETs in the plasma of normotensive WKY rats, the relative increase was less than that for the SHR and was not associated with a reduction in BP, which is not surprising when considering that most antihypertensive agents exert little or no effect on BP in normotensive individuals. The results suggest that the biological activities of trans-EETs may have contributed to the fall in the elevated BP in the SHR. There is also evidence of enhanced sensitivity of the renal vasculature to the vasodilator action of EETs in the SHR (43).
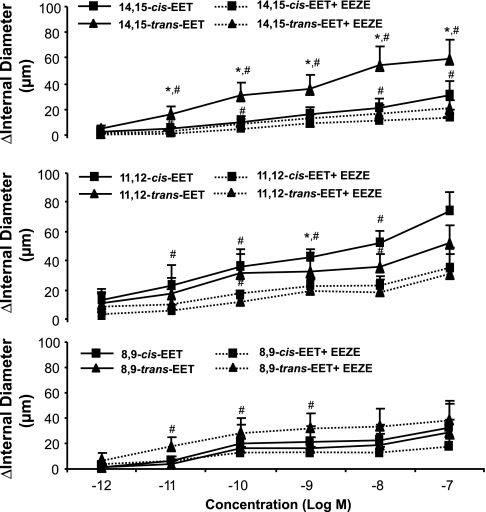
Comparison of cis- vs. trans-EETs on relaxation of preconstricted rat arcuate arteries.
Because only trans-EETs were increased by treatment with AUCB, we compared the activity of cis- and trans-EETs on a preconstricted rat arcuate artery preparation (Fig. 3). We found that the 14,15-trans-EET was more potent (ED50 5 × 10−11 M; 59 ± 15 μm dilation at 10−7M) than 14,15-cis-EET. The 11,12-EET cis- and trans-isomers were equipotent dilators (ED50 10−10 M) as were the 8,9-EET isomers (ED50 5 × 10−10 M). The vasodilator activity of EETs, except for 8,9-EETs, was significantly reduced by the presence of 14,15-EEZE (10−6 mol/l), an EET antagonist (20). The responses to 8,9-cis-EET were unaffected by preincubation with 14,15-EEZE, whereas the potency of 8,9-trans-EET was significantly enhanced in the presence of 14,15-EEZE.
Fig. 3.
Comparison of the vasoactivity of cis- and trans-EETs on pressurized rat arcuate arteries. Arteries were pressurized to 80 mmHg and preconstricted with phenylephrine. Concentration-response curves to either cis- or trans- EETs were repeated in the presence of 14,15-EEZE (EEZE). *P < 0.05 compared with responses to respective cis-EET, n = 6–8. #P < 0.05 compared with the vasodilator activity of EETs and effects of EEZE, n = 6–8.
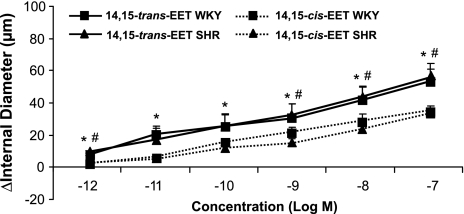
Because the 14,15-EET isomers exhibited the major difference in dilator activity, we compared the activity of 14,15-trans-EET and 14,15-cis-EET in arteries obtained from SHR and WKY rats pressurized to 80 and 120 mmHg. We observed no difference in basal diameters, sensitivity to phenylephrine, or responsiveness to EETs between SHR and WKY arteries. The 14,15-trans-EET was more potent than 14,15-cis-EET in both SHR and WKY arteries. No differences in the activities of 14,15-trans-EET and 14,15-cis-EET were apparent at 80 mmHg (Fig. 4) or 120 mmHg (data not shown).
Fig. 4.
Comparison of the vasoactivity of cis- and trans-EETs on pressurized rat arcuate arteries of 12-wk-old SHR and WKY rats. Arteries were pressurized to 80 mmHg and preconstricted with phenylephrine, and concentration-response curves to 14,15-cis- or trans-EET were performed. *P < 0.05, 14,15-trans-EET compared with responses to 14,15-cis-EET on WKY arcuate arteries, n = 4–5. #P < 0.05, 14,15-trans-EET compared with responses to 14,15-cis-EET on SHR arcuate arteries, n = 4–5.
DISCUSSION
The major new findings from this study are: 1) that plasma levels of EETs, especially trans-EETs, are reduced in the SHR; 2) the reduction in BP in SHR in response to sEH inhibition is associated with increased levels of plasma trans-EETs; and 3) the renal microvascular vasodilator activity of 14,15-trans-EET is greater than 14,15-cis-EET. In addition, the finding of increased expression and activity of sEH in RBCs of SHR compared with WKY is consistent with earlier studies showing increased sEH activity in hypertension (1, 18, 24, 55). This study also confirms that trans-EETs are hydrolyzed faster than cis-EETs by sEH (29), that inhibition of sEH lowers BP in SHR (34, 55), and that plasma EET levels are reduced in hypertension (41). When considered together, our results support the concept that an increase in plasma trans-EETs contributes to the antihypertensive effect of inhibition of sEH in SHR. Unequivocal evidence for a role of trans-EETs in the hypotensive effect of AUCB to inhibit sEH will require the demonstration that specific inhibition of the generation or activity of trans-EETs can prevent the hypotensive effect of AUCB in the SHR, a challenging task requiring elucidation of the mechanism of trans-EET formation and development of specific antagonists against the action of trans-EETs.
This study points to a potential role of RBCs, and specifically the sEH activity of RBCs, in the regulation of BP. Whether this is linked to the antihypertensive effect of phlebotomy (2, 13) or embryo transfer (33) deserves further investigation. Similarly, it would be of great interest to assess any effects on BP resulting from transfusion of RBCs from SHR to WKY rats. This present study identified increases mostly in plasma trans-EETs with AUCB treatment, but more profound increases in cis- and trans-EETs in specific vascular beds cannot be excluded.
SHR features increased oxidative stress (11, 46), and the lipid hydroperoxide-dependent CYP metabolism should result in elevated trans-EET formation. However, plasma trans-EET levels are significantly lower in SHR compared with WKY and may reflect a deficiency in the formation of trans-EETs by SHR. The preferred hydrolysis of trans-EETs by sEH may also account for the deficit of trans-EETs in the plasma of SHR vs. WKY, since sEH expression and activity are increased more than twofold in RBCs from the SHR. Consequently, we envisaged that inhibition of sEH would increase levels of trans-EETs more than those of cis-EETs and that the increase in trans-EETs would be relatively greater for the SHR. Both of these predictions proved correct; trans-EETs were increased in the plasma of both the WKY and the SHR following treatment with AUCB, whereas there was no change in total plasma cis-EETs; levels of trans-EETs in the plasma of the SHR were increased twofold compared with only a 50% increase in WKY. These results indicate a potential role for trans-EETs in the hypotensive effect of AUCB in the SHR, an idea that is further supported by the greater or equal renal vasodilator activity of trans- vs. cis-EETs (Fig. 3).
EETs have direct actions to relax vascular smooth muscle, presumably via activation of potassium channels (5, 31), and EETs may also potentiate the dilator effects of other hormones (5, 30). Consequently, an increase in trans-EETs in response to inhibition of sEH might account for the hypotensive effect of AUCB in SHR through direct and indirect mechanisms. Of the EET isomers, 14,15-trans-EET and 11,12-cis-EET were equipotent vasodilators. The 14,15-EET isomers exhibited the greatest differences in potency with the 14,15-trans-EET being a more potent renal vasodilator than 14,15-cis-EET. Falck et al. (14) have reported less potency for 14,15-trans- vs. 14,15-cis-EET in bovine coronary arteries (14), and this discrepancy should be of no surprise considering the different potencies of EETs in various vascular beds and species (5, 9, 12, 44). However, in isolated arcuate arteries obtained from SHR and WKY rats, the greater vasodilator responses to 14,15-trans- compared with 14,15-cis-EET were consistent with those obtained from normotensive SD rats, and no difference in responsiveness was observed between arteries from SHR and WKY rats. This is in contrast to the results obtained in the isolated perfused kidney of SHRs where vasodilator responses to 5,6-EET and its precursor, arachidonic acid, were markedly enhanced compared with those of control rats (42, 43). The vasodilator activity of the cis- and trans-EETs, except for 8,9-EETs, was attenuated in the presence of the putative EET antagonist 14,15-EEZE, suggesting a common mechanism for vasorelaxation. In contrast, the responses to 8,9-cis-EET were not attenuated by 14,15-EEZE, and those of 8,9-trans-EET were enhanced, suggesting an EET receptor-independent mechanism for 8,9-trans-EET that is amplified with 14,15-EEZE.
A role for cis-EETs in BP reduction in the SHR with sEH inhibition was not directly supported by this study of circulatory EETs; however, an elevation in both cis- and trans-EETs in tissues such as the kidney that are involved in BP regulation cannot be discounted. Imig et al. (24) reported decreased urinary EET excretion in rats with ANG II-induced hypertension and elevated excretion of 14,15-DHET, consistent with increased activity of sEH. Inhibition of sEH increased urinary EETs, decreased 14,15-DHET, and resulted in a diuresis that paralleled the fall in BP (24). In contrast to those results, we were unable to discern any effect of AUCB treatment of SHR and WKY rats for 7 days on the urinary excretion of EETs or DHETs, possibly signifying a lack of effect of AUCB to inhibit sEH in our study. This possibility was highly unlikely, since RBCs obtained from rats treated with AUCB showed a marked impairment in the transformation of 14,15-cis- and 14,15-trans-EET to 14,15-threo- and 14,15-erythro-DHET, respectively. Similar to the results of Imig et al. for the urinary excretion of EETs in the ANG II hypertensive rat, we found that excretion of urinary EETs in the SHR was ∼50% less than that in the WKY; however, we saw no increase in DHET excretion. It should be noted that the urinary EETs and DHETs analyzed in this study represented total amounts measured by GC/MS, which cannot distinguish between individual isomers.
The results of the present study confirm the beneficial effects of sEH inhibitors that have been reported for a variety of pathological conditions, including hypertension (45, 49), but extend these findings to invoke a novel mechanism for the hypotensive effect of AUCB that we postulate is mediated by an increase in plasma trans-EETs. The consideration of a role for RBCs in vascular regulation derives from their ability to release and hydrolyze EETs (25, 27, 28). Because RBCs are a major source of sEH in the circulation, inhibition of sEH elevated plasma EETs. This reflects a substantial increase in trans-EETs with little or no change in cis-EETs and is consistent with the preferred hydrolysis of trans- over cis-EETs by the sEH (29). Increased plasma levels of trans-EETs and greater vasodilator activity of trans- than cis-EETs may account for the reduction in BP by sEH inhibition in the SHR. Because activity of many CYP isoforms can be driven by lipid hydroperoxides (4, 40), formation of cis- and trans-EETs should be increased with enhanced endothelial expression of CYP epoxygenases, resulting in BP reduction (32). Thus, increasing CYP metabolism that consumes lipid hydroperoxides and forms trans-EETs may be a significant antioxidant and antihypertensive remedy (4, 7, 52).
GRANTS
This study was supported by grants from New York Medical College and from National Institutes of Health Grants PPG 34300, HL-25394, DK-69687, and DK-38226, and the Robert A. Welch Foundation GL-625910. Partial support was obtained from NIH Grant ES-002710.
DISCLOSURES
B. D. Hammock is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
ACKNOWLEGMENTS
We thank Dr. John C. McGiff for critical reading and valuable comments of our manuscript.
REFERENCES
- 1. Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA 104: 9018–9023, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barenbrock M, Spieker C, Rahn KH, Zidek W. Therapeutic efficiency of phlebotomy in posttransplant hypertension associated with erythrocytosis. Clin Nephrol 40: 241–243, 1993 [PubMed] [Google Scholar]
- 3. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 4. Bui PH, Hsu EL, Hankinson O. Fatty acid hydroperoxides support cytochrome P450 2S1-mediated bioactivation of benzo[a]pyrene-7,8-dihydrodiol. Mol Pharmacol 76: 1044–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch 459: 881–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell WB, Gauthier KM. What is new in endothelium-derived hyperpolarizing factors? Curr Opin Nephrol Hypertens 11: 177–183, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Capdevila J, Saeki Y, Falck JR. The mechanistic plurality of cytochrome P-450 and its biological ramifications. Xenobiotica 14: 105–118, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Capdevila JH, Karara A, Waxman DJ, Martin MV, Falck JR, Guenguerich FP. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem 265: 10865–10871, 1990 [PubMed] [Google Scholar]
- 9. Carroll MA, Schwartzman M, Capdevila J, Falck JR, McGiff JC. Vasoactivity of arachidonic acid epoxides. Eur J Pharmacol 138: 281–283, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys 223: 639–648, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 293: H3246–H3253, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol 141: 441–448, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fahal IH, Yaqoob M, Ahmad R. Phlebotomy for erythropoietin-induced malignant hypertension. Nephron 61: 214–216, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, Campbell WB. Comparison of vasodilatory properties of 14,15-EET analogs: structural requirements for dilation. Am J Physiol Heart Circ Physiol 284: H337–H349, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21: 1288–1291, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Fitzpatrick FA, Ennis MD, Baze ME, Wynalda MA, McGee JE, Liggett WF. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry. J Biol Chem 261: 15334–15338, 1986 [PubMed] [Google Scholar]
- 18. Fornage M, Hinojos CA, Nurowska BW, Boerwinkle E, Hammock BD, Morisseau CH, Doris PA. Polymorphism in soluble epoxide hydrolase and blood pressure in spontaneously hypertensive rats. Hypertension 40: 485–490, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 86: 6377–6381, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh S, Chiang PC, Wahlstrom JL, Fujiwara H, Selbo JG, Roberds SL. Oral delivery of 1,3-dicyclohexylurea nanosuspension enhances exposure and lowers blood pressure in hypertensive rats. Basic Clin Pharmacol Toxicol 102: 453–458, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Goulitquer S, Dreano Y, Berthou F, Corcos L, Lucas D. Determination of epoxyeicosatrienoic acids in human red blood cells and plasma by GC/MS in the NICI mode. J Chromatogr B Analyt Technol Biomed Life Sci 876: 83–88, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem 50: 3825–3840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39: 690–694, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Jiang H, Anderson GD, McGiff JC. Red blood cells (RBCs), epoxyeicosatrienoic acids (EETs) and adenosine triphosphate (ATP). Pharmacol Rep 62: 468–474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang H, McGiff JC, Quilley J, Sacerdoti D, Reddy LM, Falck JR, Zhang F, Lerea KM, Wong PY. Identification of 5,6-trans-epoxyeicosatrienoic acid in the phospholipids of red blood cells. J Biol Chem 279: 36412–36418, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Jiang H, Quilley J, Reddy LM, Falck JR, Wong PY, McGiff JC. Red blood cells: reservoirs of cis- and trans-epoxyeicosatrienoic acids. Prostaglandin Lipid Mediat 75: 65–78, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X(7) receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol 151: 1033–1040, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang H, Zhu AG, Mamczur M, Morisseau C, Hammock BD, Falck JR, McGiff JC. Hydrolysis of cis- and trans-epoxyeicosatrienoic acids by rat red blood cells. J Pharmacol Exp Ther 326: 330–337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci 28: 32–38, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol 290: H491–H499, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J 24: 3770–3781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JY, Azar SH. Wistar-Kyoto and spontaneously hypertensive rat blood pressure after embryo transfer into different wombs and cross-suckling. Exp Biol Med (Maywood) 235: 1375–1384, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci 13: 3480–3487, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Liclican EL, Doumad AB, Wang J, Li J, Falck JR, Stier CT, Jr, Carroll MA. Inhibition of the adenosine2A receptor-epoxyeicosatrienoic acid pathway renders Dahl salt-resistant rats hypertensive. Hypertension 54: 1284–1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin WK, Falck JR, Wong PY. Effect of 14,15-epoxyeicosatrienoic acid infusion on blood pressure in normal and hypertensive rats. Biochem Biophys Res Commun 167: 977–981, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Loch D, Hoey A, Morisseau C, Hammock BO, Brown L. Prevention of hypertension in DOCA-Salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys 47: 87–98, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2414–2420, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol 297: F740–F748, 2009. 19553349 [Google Scholar]
- 40. McCallum GP, Weedon AC, Krug P, Bend JR. Microsomal cytochrome P450 peroxygenase metabolism of arachidonic acid in guinea pig liver. J Pharmacol Exp Ther 278: 1188–1194, 1996 [PubMed] [Google Scholar]
- 41. Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension 51: 1379–1385, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pomposiello SI, Carroll MA, Falck JR, McGiff JC. Epoxyeicosatrienoic acid-mediated renal vasodilation to arachidonic acid is enhanced in SHR. Hypertension 37: 887–893, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6-Epoxyeicosatrienoic acid mediates the enhanced renal vasodilation to arachidonic acid in the SHR. Hypertension 42: 548–554, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Pratt PF, Li P, Hillard CJ, Kurian J, Campbell WB. Endothelium-independent, ouabain-sensitive relaxation of bovine coronary arteries by EETs. Am J Physiol Heart Circ Physiol 280: H1113–H1121, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Revermann M. Pharmacological inhibition of the soluble epoxide hydrolase-from mouse to man. Curr Opin Pharmacol 10: 173–178, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA 106: 5171–5176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sellers KW, Sun C, Diez-Freire C, Waki H, Morisseau C, Falck JR, Hammock BD, Paton JF, Raizada MK. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J 19: 626–628, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Shen HC. Soluble epoxide hydrolase inhibitors: a patent review. Expert Opin Ther Pat 20: 941–956, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res Suppl 50: S52–S56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43: 55–90, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Vaz AD, McGinnity DF, Coon MJ. Epoxidation of olefins by cytochrome P450: evidence from site-specific mutagenesis for hydroperoxo-iron as an electrophilic oxidant. Proc Natl Acad Sci USA 95: 3555–3560, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, Kroetz DL, Weiss RH. Vascular localization of soluble epoxide hydrolase in the human kidney. Am J Physiol Renal Physiol 286: F720–F726, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Zeldin DC, DuBois RN, Falck JR, Capdevila JH. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys 322: 76–86, 1995 [DOI] [PubMed] [Google Scholar]