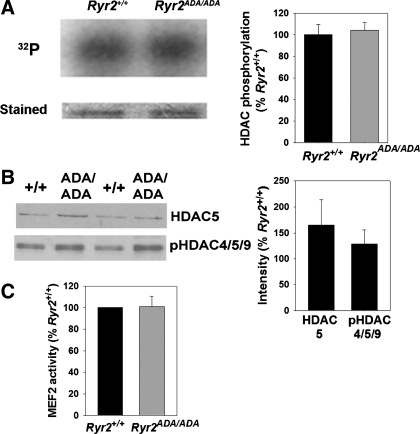
Fig. 2.
Histone deacetylase (HDAC) phosphorylation and myocyte enhancer factor 2 (MEF2) activities in hearts of E16.5 Ryr2+/+ and Ryr2ADA/ADA mice. A: [32P] incorporation (left, top) and Coomassie brilliant blue staining (left, bottom) of GST-HDAC4 fusion protein (amino acids 419–670). GST-HDAC4 fusion protein was a substrate for kinase activities of heart lysates from E16.5 Ryr2+/+ and Ryr2ADA/ADA mice. [32P]radioactivity in GST-HDAC4 SDS-PAGE gel bands was determined by liquid scintillation counting (right). Data are means ± SE of 4 to 5 heart lysates. B: immunoblot analysis of HDAC5 and pHDAC4-Ser246/HDAC5-Ser259/HDAC9-Ser220. Aliquots of heart homogenates from E16.5 Ryr2+/+ and Ryr2ADA/ADA mice were separated by 10% SDS/PAGE, transferred to nitrocellulose membranes, and probed with rabbit polyclonal antibodies (left). Intensities of protein bands of E16.5 Ryr2ADA/ADA were normalized by comparing them with respective E16.5 Ryr2+/+ bands. GAPDH was used as loading control. Data are means ± SE of 6 WT and mutant samples each. C: MEF2 activity of heart lysates from E16.5 Ryr2+/+ and Ryr2ADA/ADA mice. HDAC4 kinase and MEF2 activities were normalized to those of Ryr2+/+ (WT). Data are means ± SE of experiments with 4 to 5 heart lysates.