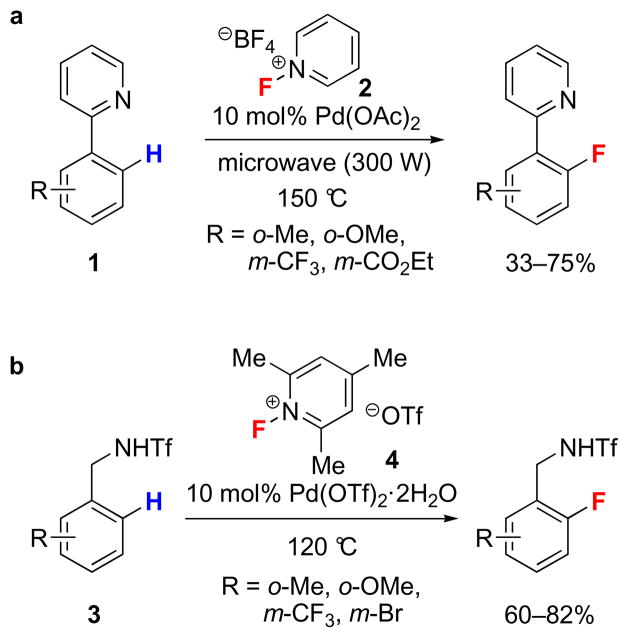
Figure 1. Directed electrophilic palladium-catalyzed Ar–F bond-forming reactions.
a, The first palladium-catalyzed fluorination of organic molecules. Phenylpyridine derivatives (1) were fluorinated in the presence of 10 mol% of Pd(OAc)2 and the electrophilic fluorination reagent N-fluoropyridinium tetrafluoroborate (2) under microwave irradiation. b, A palladium-catalyzed directed electrophilic fluorination of C–H bonds of N-benzyltriflamide derivatives (3) with the catalyst Pd(OTf)2·2H2O and the electrophilic fluorination reagent N-fluoro-2,4,6-trimethylpyridinium triflate (4). (Ac: acetyl, Me: methyl, Et: ethyl, Tf: trifluoromethanesulfonyl).