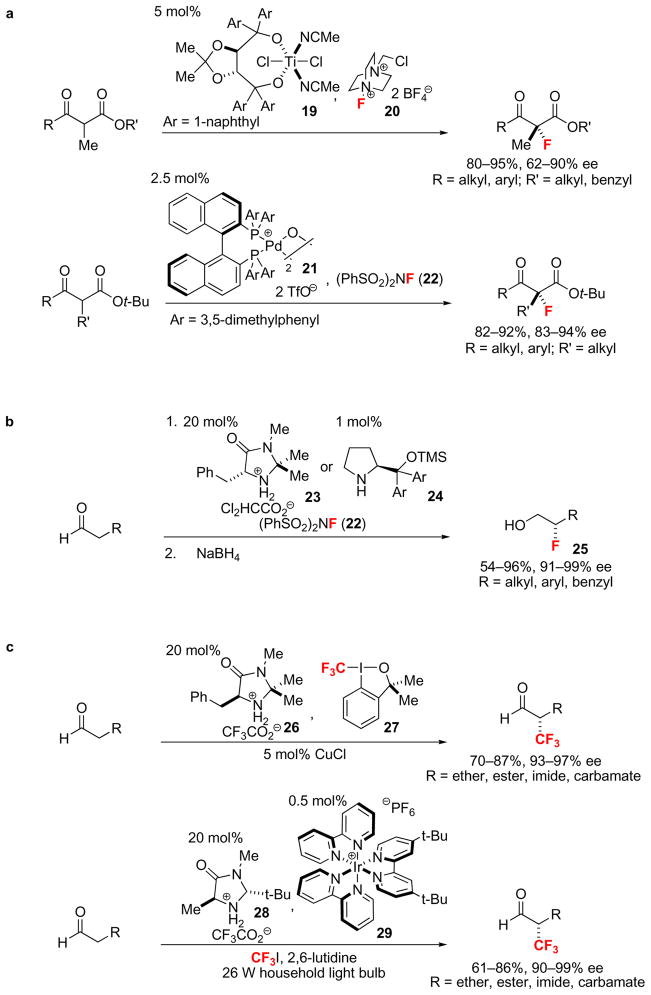
Figure 5. Catalytic enantioselective Csp3–F and Csp3–CF3 bond-forming reactions.
a, Metal-catalyzed enantioselective Csp3–F bond-forming reactions. Branched β-ketoesters were fluorinated using 5 mol% of a Ti-TADDOL catalyst (19) and Selectfluor® (20) or 2.5 mol% of μ-hydroxo-palladium-BINAP complex (21) and N-fluorobenzenesulfonimide (22). b, Examples of organocatalytic enantioselective Csp3–F bond-forming reactions. Amino acid-derived organocatalysts (23 and 24) and N-fluorobenzenesulfonimide (22) were utilized to fluorinate α-unbranched aldehydes. Due to the potentially facile racemization of α-fluoroaldehydes, the corresponding fluorohydrins (25) were isolated after reduction with NaBH4 in 54–96% yield and 91–99% ee. c, Enantioselective Csp3–CF3 bond-forming reactions. The mechanism of the two presented reactions differ conceptually, but both afford α-trifluoromethylated aldehydes in good yield and high enantioselectivity either using hypervalent iodine 27 as the CF3 source and amine catalyst 26 or using trifluoroiodomethane as the CF3 source, 20 mol% amine catalyst 28, 0.5 mol% Ir catalyst 29 and light. (TMS: trimethylsilyl).