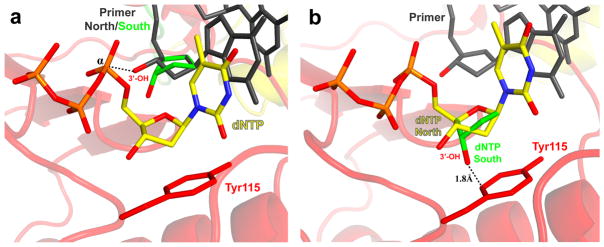
Figure 2.
The effect of sugar ring conformation in the HIV-1 RT polymerase active site. The sugar ring conformation at the 3’-primer end is required to be in the North (2’-exo/3’-endo) conformation (2a, dark gray) for successful in-line nucleophilic attack of the α-phosphate of the incoming dNTP or NRTI. The South (2’-endo/3’-exo) (2a, green) conformation of the sugar ring at the primer terminus positions the 3’-OH away from the α-phosphate and thus DNA polymerization is not as efficient. The preferred conformation of the sugar ring of the incoming dNTP or NRTI is the North conformation (2b, yellow). The South conformation of the sugar ring of the incoming dNTP or NRTI (2b, green) would result in a very short distance between the 3’-OH and the aromatic ring of Tyr115 (red), causing steric interactions with the enzyme. Coot (10) was used to perform simple modeling of the 2’-endo/3’-exo sugar ring conformations of the primer and dNTP using structural coordinates from PDB Code 1RTD. Images were generated using PyMOL (7).