Abstract
Although the neuropilins were characterized as semaphorin receptors that regulate axon guidance, they also function as vascular endothelial growth factor (VEGF) receptors and contribute to the development of other tissues. Here, we assessed the role of NRP2 in mouse mammary gland development based on our observation that NRP2 is expressed preferentially in the terminal end buds of developing glands. A floxed NRP2 mouse was bred with an MMTV-Cre strain to generate a mammary gland-specific knockout of NRP2. MMTV-Cre;NRP2loxP/loxP mice exhibited significant defects in branching morphogenesis and ductal outgrowth compared with either littermate MMTV-Cre;NRP2+/loxP or MMTV-Cre mice. Mechanistic insight into this morphological defect was obtained from a mouse mammary cell line in which we observed that VEGF165, an NRP2 ligand, induces branching morphogenesis in 3D cultures and that branching is dependent upon NRP2 as shown using shRNAs and a function-blocking antibody. Epithelial cells in the mouse mammary gland express VEGF, supporting the hypothesis that this NRP2 ligand contributes to mammary gland morphogenesis. Importantly, we demonstrate that VEGF and NRP2 activate focal adhesion kinase (FAK) and promote FAK-dependent branching morphogenesis in vitro. The significance of this mechanism is substantiated by our finding that FAK activation is diminished significantly in developing MMTV-Cre;NRP2loxP/loxP mammary glands compared with control glands. Together, our data reveal a VEGF/NRP2/FAK signaling axis that is important for branching morphogenesis and mammary gland development. In a broader context, our data support an emerging hypothesis that directional outgrowth and branching morphogenesis in a variety of tissues are influenced by signals that were identified initially for their role in axon guidance.
Keywords: Mammary gland, Neuropilin, Branching morphogenesis, FAK, Mouse
INTRODUCTION
The neuropilins (NRP1 and NRP2) were identified as receptors for the semaphorin family of axon guidance molecules and they are crucial for development of the nervous system (Kolodkin et al., 1997; He and Tessier-Lavigne, 1997). The neuropilins can also bind a specific isoform of vascular endothelial growth factor (VEGF165) (Soker et al., 1998) and function as co-receptors for tyrosine kinase VEGF receptors (Neufeld et al., 2002), and possibly other growth factor and integrin receptors (Hu et al., 2007; Matsushita et al., 2007; Sulpice et al., 2008). Although NRP1 and NRP2 are structurally homologous (44% amino acid identity), they differ in their regulation and function. For example, we reported that metabolic stress induces the lysosomal degradation of NRP1 but not NRP2 (Bae et al., 2008). The NRPs have been studied extensively for their involvement in neural development and to some extent vascular development, but their potential contribution to the development of other tissues has not been investigated extensively. This issue is important because there is increasing evidence that NRPs influence the behavior of carcinoma cells and might be potential therapeutic targets (Bagri et al., 2009). An understanding of the potential role of NRPs in epithelial development and homeostasis would provide mechanistic insight into their role in carcinomas.
Our interest is in the function of the NRPs in mammary gland biology and breast cancer. NRP1 and NRP2 are expressed in the mammary gland and in breast cancers (Bagri et al., 2009; Morris et al., 2006; Uniewicz and Fernig, 2008), but their functional roles are still being elucidated. Previous studies by our group and others demonstrated the importance of NRP1 in the migration and survival of breast carcinoma cells (Bachelder et al., 2001; Bachelder et al., 2002; Bachelder et al., 2003; Barr et al., 2005; Castro-Rivera et al., 2004; Timoshenko et al., 2007). Recent data indicate that NRP2 expression in breast cancer correlates with lymph node metastasis and poor prognosis suggesting that it also regulates key functions associated with breast carcinoma progression (Yasuoka et al., 2009). Despite these interesting observations in breast cancer, nothing is known about the potential role of the NRPs in mammary gland development. Although Nrp2−/− mice are viable, females produce litters with few offspring (Giger et al., 2000; Chen et al., 2000). To assess the potential contribution of NRP2 to mammary gland development, we generated mice with a targeted deletion of NRP2 in the mammary gland. Analysis of mammary gland development in these mice revealed a marked defect in branching morphogenesis and ductal outgrowth, but no evidence of an epithelial-mesenchymal transition (EMT) in terminal end buds (TEBs). Mechanistic insight into this morphological defect was obtained from a mouse mammary gland cell line in which we observed that VEGF165, an NRP2 ligand, induces branching morphogenesis in 3D cultures and that branching is dependent upon NRP2 expression. Importantly, we observed that VEGF and NRP2 stimulate activation of focal adhesion kinase (FAK; PTK2 – Mouse Genome Informatics), which has been implicated in mammary gland branching and development (Nagy et al., 2007; Provenzano et al., 2008; van Miltenburg et al., 2009). Moreover, FAK activation is diminished significantly in developing MMTV-Cre;NRP2loxP/loxP mammary glands compared with control glands.
MATERIALS AND METHODS
NRP2 knockout mice
Male C57BL/6 NRP2+/loxP mice were obtained from Alex Kolodkin at Johns Hopkins University with permission from Peter Mombaerts (Walz et al., 2002). Female C57BL/6 MMTV-Cre mice (Wagner et al., 1997) were used to generate MMTV-Cre;NRP2loxP/loxP mice. Three to four mice per cage were housed under a standard 12-hour photoperiod. Food and water were provided ad libitum. All of the animal breeding and procedures were approved by the Institutional Animal Care and Use Committee of University of Massachusetts Medical School.
Mammary gland preparation
One of the fourth inguinal mammary glands from 5-, 7-, 9- and 12-week-old virgin mice was whole mounted for carmine staining and the other was embedded in paraffin after formalin fixation for immunohistochemistry (IHC) and Hematoxylin and Eosin (H&E) staining. The fifth glands and adjacent skin were also removed for freezing and paraffin embedding. Carmine-stained virgin mammary glands (wild type C57BL/6, 7 weeks old) were used to isolate TEBs and mature ducts under a dissecting microscope to compare relative NRP expression. To verify NRP2 ablation in mammary glands, the central area of the fourth mammary gland (right side) from 5-week-old virgin MMTV-Cre;NRP2loxP/loxP mice was compared with those of littermate MMTV-Cre;NRP2+/loxP and MMTV-Cre mice for NRP mRNA expression. The remaining part of the right mammary gland and the entire left mammary gland were whole-mounted to examine the developmental status of the mammary gland.
Quantification of mouse mammary gland development
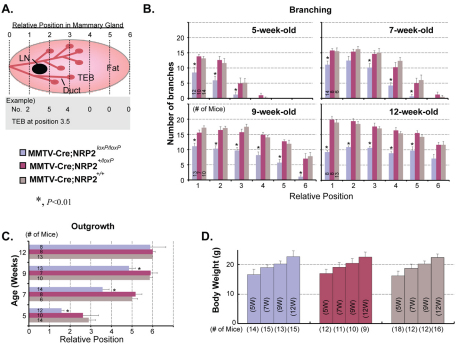
For analyzing relative mammary gland development, branching density and growth rate of mammary glands into the fat pad were compared between the fourth inguinal mammary glands of MMTV-Cre;NRP2loxP/loxP mice and those of littermate female MMTV-Cre;NRP2+/loxP and MMTV-Cre or wild-type (control) mice (age-matched NRP2+/flox, NRP2flox/flox and wild-type virgin mice). For each experimental group, 6-18 mice were used. Each scanned image of whole-mounted mammary glands was aligned and divided with the same six imaginary lines by positioning their central lymph nodes one quarter of the way along from the left of the entire mammary gland (Fig. 2A).
Fig. 2.
Loss of NRP2 impedes branching morphogenesis and invasion of epithelial cells into the mammary fat pad. (A) Schematic of method for quantification of mouse mammary gland development. Each whole-mounted mammary gland was divided into six areas by positioning their central lymph nodes at position 1.5 from the left (central line of the body). The number of mammary ducts crossing each line, ductal outgrowth and body weight were determined for quantification of relative mammary gland development. MMTV-Cre;NRP2loxP/loxP mice were compared with MMTV-Cre;NRP2+/loxP or MMTV-Cre mice for branching density and mammary gland outgrowth, as well as body weight. For each experimental group, 6-18 mice were analyzed at each of four developmental stages (5, 7, 9 and 12 weeks old). LN, central lymph node; TEB, terminal end bud. (B) Effect of NRP2 loss on branching density. (C) Effect of NRP2 loss on mammary gland outgrowth. (D) Body weights were compared as an index for overall development. All experimental groups exhibited similar rates of body weight increase with age. For all graphs, error bars represent s.e.m., *P<0.05.
Immunohistochemistry
Antibodies (Abs, 1 μg/ml) specific for either E-cadherin (4A2C7, Invitrogen), Ki67 (NCL-Ki67p, Leica Microsystems), phospho-Tyr 397-FAK (p-FAK, Biosource) or VEGF (Ab46154, Abcam) were used for immunohistochemistry. Briefly, 5-week-old mammary gland sections on Superfrost Plus microscope slides (48312-501, VWR International) were steamed for 30 minutes in a citrate buffer (pH 6.0; Zymed/Invitrogen, Carlsbad, CA, USA) for antigen retrieval. Endogenous peroxidase was quenched using 3% hydrogen peroxide (Sigma-Aldrich, St Louis, MO, USA). Endogenous biotin was blocked using the avidin/biotin blocking system (Vector Laboratories, Burlingame, CA, USA). For the rabbit polyclonal Abs (Ki67, p-FAK and VEGF), casein (Vector Laboratories) was used to reduce non-specific staining. The M.O.M. Kit (PK-2200, Vector Laboratories) blocking agent was used for the mouse monoclonal Ab E-cadherin. IHC detection was performed with the appropriate biotinylated secondary Ab, a Vectastain ABC Elite Kit and 3,3′-diaminobenzidine (Vector Laboratories). For E-cadherin IHC, the biotinylated mouse secondary Ab from the M.O.M. Kit was used with the Vectastain ABC Elite Kit and 3,3′-diaminobenzidine. The same serial sections were stained with H&E before antigen retrieval for observing overall structures of TEBs and ducts of each mouse.
Cell cultures
Immortalized normal mouse mammary gland epithelial cells (NMuMG cells) were purchased from the ATCC and maintained in DMEM (high glucose) containing 10% fetal bovine serum, 10 μg/ml insulin and 1% streptomycin and penicillin at 37°C in an incubator supplied with 5% CO2. To induce EMT, human recombinant transforming growth factor β1 (TGFB1 – Human Gene Nomenclature Database; TGFβ, 5 ng/ml, PeproTech) was added to NMuMG cells. VEGF165 (10 ng/ml, PeproTech) or fibroblast growth factor 2 (10 ng/ml FGF2 or bFGF, PeproTech, Rocky Hill, NJ, USA) were also used to stimulate NMuMG cells. For the TGFβ-withdrawal experiments, NMuMG cells that had been treated with TGFβ for 7 days were passed into fresh medium without TGFβ for the indicated time periods. For 3D Matrigel cultures, a base layer of Matrigel (BD Bioscience CB-40230; 200 μl/well) was overlaid in duplicate wells of a 24-well dish with 1.0×104 cells suspended in 300 μl of a 2:1 mixture of PBS and Matrigel. The Matrigel was overlaid with complete serum-containing medium (0.5 ml/well), which was changed every three days. Growth factors were added to these cultures as described above. For inhibition experiments, either an NRP2 Ab (AF2215; R&D Systems) or goat IgG (005-000-003; Jackson ImmunoResearch) at a concentration of 0.2 μg/ml was added to the medium containing VEGF. Images were captured and analyzed using SPOT image analysis software (Molecular Diagnostics). Branching was quantified by counting the number of colonies with branching at day 15 of Matrigel culture and expressing this value as a percentage of the total number of colonies in four independent experiments. The data are reported as the fold-change in branching for each experimental condition relative to the appropriate control. The image analysis software was also used to measure the diameter of the colonies in the same experiments with a minimum of 47 colonies analyzed for each experimental condition. The data are reported as the fold-change in diameter for each experimental condition relative to the appropriate control. In some experiments, a constitutively active FAK plasmid (K38A, a gift of Jun-Lin Guan, University of Michigan, MI, USA) or vector alone were transfected in NMuMG cells using a Nucleofector kit (Lonza). A GFP plasmid was included in each experiment to assess transfection efficiency.
RNAi
Mouse NRP2 siRNAs (100 nM, On-TARGETplus SMARTpool siRNA, Dharmacon; L-040423-00) were used to deplete NRP2 expression in NMuMG cells based on the manufacturer's instructions. A non-targeting siRNA pool (Dharmacon D-001810-10) was used as a control for these experiments. Lentiviruses containing NRP2 shRNAs (Open Biosystems; clone ID TRCN0000063310 or TRCN0000028976) or a GFP control (RHS4459) were generated, titrated according to the manufacturer's instructions and used to infect NMuMG cells following standard protocols. Stable cell transfectants were generated by puromycin selection (2 μg/ml).
Immunoblotting
Total cellular proteins were extracted in RIPA buffer (Boston Bioproducts, Worcester, MA, USA) containing protease inhibitors (Complete Mini, Roche, Indianapolis, IN, USA), cleared by centrifugation, and quantified using the Bradford assay (BioRad, Hercules, CA, USA). For 3D Matrigel cultures, cells were photographed and branching was quantified. Subsequently, the medium was removed and 300 μl of dispase (BD Transduction) was added for 2 hours at 37°C. Alternatively, cells were isolated using Cell Resuspension solution (BD Transduction) for p-FAK or FAK immunoblotting. Blots were incubated at 4°C overnight with Abs specific for either NRP2 (AF2215, R&D Systems), NRP1 (A12, Santa Cruz), E-cadherin (4A2C7, Invitrogen), N-cadherin (32, Invitrogen), β-actin (Sigma-Aldrich), p-FAK (pY397) (BD) or total FAK (C-20, Santa Cruz) and immune complexes were detected using enhanced chemiluminescence (ECL; Pierce, Rockford, IL, USA). In some experiments, NMuMG cells were serum-deprived overnight, stimulated with or without VEGF for 30 minutes, and immunoblotted for p-FAK or FAK.
Real-time RT-PCR
To assess the expression of NRPs by quantitative real-time RT-PCR (qPCR), total RNA (0.2 μg) extracted from mammary glands using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) was reverse transcribed using Reverse Transcription Reagents (Applied Biosystems) and analyzed by Taqman Universal PCR Master Mix (Applied Biosystems) using a real-time PCR System (ABI Prism 7900HT Sequence Detection System) according to the manufacturer's protocol. The primers used were: NRP2 (Hs00187290_m1), NRP1 (Hs00818574_m1) and GAPDH (Hs99999905_m1). The expression of NRP1 and NRP2 was normalized with the respective GAPDH result and analyzed by the comparative cycle threshold method (ΔΔCt). Product specificity was examined by dissociation curve analysis.
RESULTS
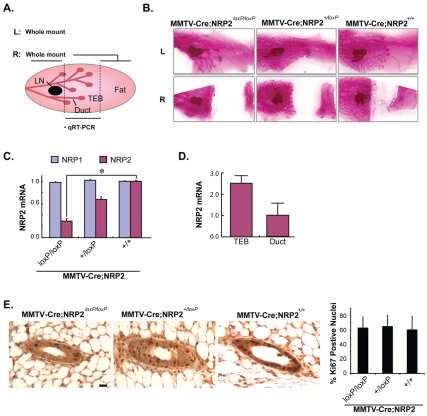
NRP2 is required for proper branching morphogenesis in the developing mouse mammary gland
The postnatal development of the mammary gland requires systemic hormones and local growth factors to induce ductal outgrowth and branching (Cowin and Wysolmerski, 2010). More specifically, structures termed terminal end buds (TEBs) form at the tips of ducts. These TEBs proliferate and ramify into the adipose tissue to form the branched structure of the mammary gland. To investigate the role of NRP2 in pubertal mammary gland development, a floxed NRP2 mouse strain was bred with an MMTV-Cre strain. The central area of the fourth inguinal mammary glands of 5-week-old virgin mice was used for verification of NRP2 knockout by quantitative real time RT-PCR (qPCR). Carmine-stained whole mounts, which were prepared with the remaining parts of that mammary gland and the other fourth gland, were used to evaluate glandular morphology and development (Fig. 1A). Analysis of whole-mounted MMTV-Cre;NRP2loxP/loxP mammary outgrowths revealed less branching morphogenesis and slower invasion of mammary ducts into the fat pads compared with littermate MMTV-Cre;NRP2+/loxP or MMTV-Cre glands (Fig. 1B and see Fig. S1 in the supplementary material). NRP2 expression in the central area of the mammary gland was significantly decreased in MMTV-Cre;NRP2loxP/loxP mice compared with MMTV-Cre;NRP2+/loxP or MMTV-Cre mice (Fig. 1C). By contrast, expression of NRP1 mRNA did not differ significantly among the genotypes. A role for NRP2 in ductal outgrowth is also substantiated by our observation that microdissected TEBs from wild-type mice expressed significantly more NRP2 mRNA than did mature ducts from the same glands (Fig. 1D). The retardation of ductal development observed in the MMTV-Cre;NRP2loxP/loxP mice was not caused by a role of NRP2 in cell proliferation as evidenced by the observation that Ki67 staining in both ducts and TEBs did not differ significantly at 5 weeks among the three genotypes (Fig. 1E).
Fig. 1.
Loss of NRP2 retards mammary gland development. (A) Schematic of mouse mammary gland preparation. Left (L) and right (R) sides of the fourth mammary glands were prepared for each mouse. Extracts prepared from the central area of the right mammary gland were used for qRT-PCR to verify loss of NRP2. The remaining parts of these mammary glands were whole-mounted as shown in B. LN, central lymph node; TEB, terminal end bud. (B) Whole mounts (carmine staining) of 5-week-old virgin mouse mammary glands. Top and bottom pictures show left (L) and right (R) side of the fourth mammary gland. (C) NRP1 and NRP2 mRNA expression in the different genotypes assessed by qPCR. Data were normalized to GAPDH for each sample and expressed as mean±s.d. *P<0.01. (D) Relative NRP2 expression in TEB and mature ducts microdissected from normal mouse mammary glands. Data were normalized to GAPDH for each sample and expressed as mean±s.d. (E) Cell proliferation in mouse mammary glands from 5-week-old MMTV-Cre;NRP2loxP/loxP, MMTV-Cre;NRP2+/loxP and MMTV-Cre mice was compared using Ki67 staining. A minimum of five mice and 30 glands were examined for each genotype. Quantification of Ki67-positive nuclei is shown in the accompanying graph. Error bars represent s.d. Scale bar: 10 μm.
To quantify the impact of NRP2 ablation on mammary gland development, the lymph node was noted for each gland and the gland was partitioned into six equal sections with the lymph node located at position 1.5 (see Fig. 2A). The number of branches crossing each of the six arbitrary lines and the extent of ductal outgrowth were determined (Fig. 2A). This analysis revealed that homozygous deletion of NRP2 significantly decreased the number of branches crossing each line in 5-, 7-, 9- and 12-week-old MMTV-Cre;NRP2loxP/loxP mammary glands compared with MMTV-Cre;NRP2+/loxP or other controls (Fig. 2B). Ductal outgrowth into the mammary fat pad was also decreased significantly in 5-, 7- and 9-week-old MMTV-Cre;NRP2loxP/loxP glands compared with MMTV-Cre;NRP2+/loxP or MMTV-Cre mice (Fig. 2C). Although the extent of ductal elongation was similar among the genotypes by 12 weeks (Fig. 2C), there was still a significant decrease in branching in the MMTV-Cre;NRP2loxP/loxP glands (Fig. 2B). No differences in overall body weight were evident among the genotypes at any of the developmental stages analyzed (Fig. 2D), discounting the possibility that the observed effects can be attributed to an indirect effect on body weight. Together, the results indicate that NRP2 is required for proper branching morphogenesis of mouse mammary glands and invasion of mammary ducts into fat pads.
Branching morphogenesis in 3D culture is mediated by VEGF165 and requires NRP2
We used NMuMG cells, which are immortalized, normal mouse mammary gland epithelial cells (Yang et al., 2009), to study the potential role of NRP2 in branching morphogenesis and mammary gland development in more detail. Initially, we focused on a possible connection between branching morphogenesis and an EMT based on previous expression analyses of branching morphogenesis in the mouse mammary gland, in which the EMT-inducing transcription factors Snail (Snai1–Mouse Genome Informatics) and Twist were found to be upregulated more in TEBs than in mature ducts (Kouros-Mehr and Werb, 2006). Interestingly, we observed that TGF-β-induced EMT of NMuMG cells induces NRP2 expression and that NRP2 is required for this EMT (see Fig. S2 in the supplementary material). Based on these findings, we analyzed the mammary glands from MMTV-Cre, MMTV-Cre;NRP2+/loxP and MMTV-Cre;NRP2loxP/loxP mice for evidence of EMT by morphology and E-cadherin expression using immunohistochemistry (IHC). As shown in Fig. S3 in the supplementary material, E-cadherin is expressed in the TEBs of all three genotypes. Semi-quantitative analysis of this staining revealed no significant difference in E-cadherin expression in the ducts and TEBs among the three genotypes analyzed. Moreover, an epithelial morphology was observed in the TEBs and there was no evidence of a mesenchymal transition in any genotype (see Fig. S3 in the supplementary material).
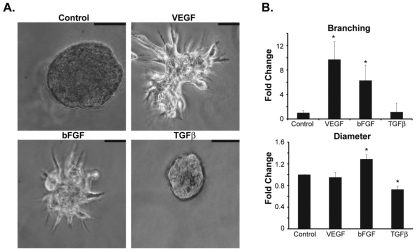
Given the results above, we used 3D Matrigel cultures to evaluate the role of NRP2 in branching morphogenesis more rigorously. NMuMG cells form spheroids when grown in Matrigel, similar to other epithelial cells. Surprisingly, TGF-β, which induces significant morphological changes in 2D cultures (see Fig. S2 in the supplementary material), had little effect on the morphology of NMuMG spheroids in 3D cultures and it actually reduced the diameter of these spheroids significantly (Fig. 3A,B). In marked contrast, VEGF165 induced extensive outgrowth and branching in 3D cultures (Fig. 3A,B), without showing any effect on morphology in 2D cultures (see Fig. S2 in the supplementary material). We also observed that bFGF, which is known to be involved in mammary gland development, stimulated branching in 3D Matrigel cultures (Fig. 3A,B). These results substantiate the importance of the microenvironment in response to growth factors and branching morphogenesis.
Fig. 3.
VEGF165 and bFGF induce branching morphogenesis of mouse mammary epithelial cells in 3D Matrigel cultures. (A) NMuMG cells were grown for 15 days in 3D Matrigel cultures in the presence of either VEGF165 (10 ng/ml), bFGF (10 ng/ml) or TGFβ (5 ng/ml). Scale bars: 50 μm. (B) The number of branched spheroids was quantified and the data are presented as the fold-change relative to untreated (control) cells. The diameter of each spheroid was also measured and the data are reported as the fold-change relative to untreated (control) cells. Error bars represent s.d., *P<0.01.
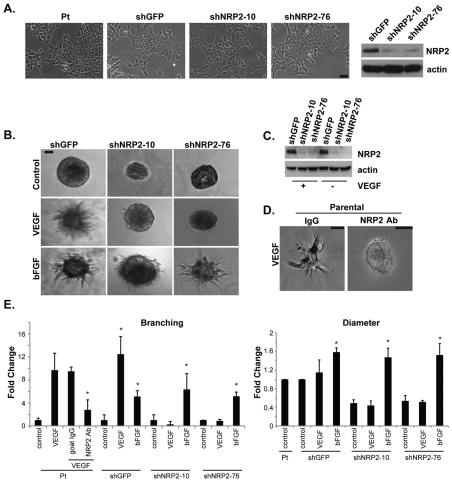
To determine the involvement of NRP2 in outgrowth and branching of NMuMG cells in 3D cultures, we generated transfectants with a stable depletion of NRP2 using two independent shRNAs (Fig. 4A). Depletion of NRP2 expression had no effect on cell morphology in 2D cultures (Fig. 4A). Cells with diminished NRP2 expression by shRNA, however, were unable to undergo outgrowth and branching in 3D Matrigel cultures in response to VEGF165 (Fig. 4B,C,E). The shRNA data were substantiated using a function-blocking NRP2 Ab, which impeded branching significantly in comparison with control IgG (Fig. 4D,E). Interestingly, loss of NRP2 decreased colony size (diameter) in Matrigel but these colonies were healthy because they exhibited branching morphogenesis and normal colony size in response to bFGF stimulation (Fig. 4B,E).
Fig. 4.
VEGF165-induced branching is dependent on NRP2. (A) NRP2 expression was depleted in NMuMG cells using two independent shRNAs (shNRP-10 and shNRP-76) and compared with a control shRNA (shGFP). Both shRNAs depleted NRP2 expression, as shown in the immunoblot, but they did not affect the morphology of NMuMG cells in 2D culture as evidenced by the photomicrographs shown. (B) Diminished NRP2 expression inhibits the ability of NMuMG cells to undergo branching morphogenesis in response to VEGF165 but not bFGF. (C) NMuMG cells that express the indicated shRNAs were cultured in Matrigel for 15 days in the presence or absence of VEGF165 and immunoblotted for NRP2 and actin. Note that the loss of NRP2 expression caused by shRNA is maintained in long-term Matrigel culture. (D) The shRNA data were substantiated using an NRP2 function-blocking antibody (0.2 μg/ml), which inhibited branching significantly in comparison with control IgG. (E) Branching and colony size (diameter) were quantified from multiple experiments using image analysis software. This analysis confirms that loss of NRP2 expression inhibits the ability of VEGF165 but not bFGF to induce branching. Interestingly, loss of NRP2 diminished colony size but these colonies were healthy because they exhibited branching morphogenesis and normal colony size in response to bFGF stimulation. The asterisks indicate significant differences (P<0.05) between the experimental condition and the appropriate control for that condition. Pt, parental.
VEGF/NRP2 signaling activates FAK, which contributes to branching morphogenesis
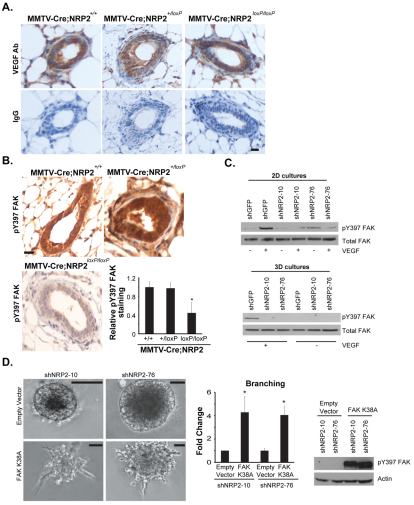
A potential role for VEGF in outgrowth and branching of the mammary gland is novel. This hypothesis assumes, however, that VEGF is present in epithelial cells of the developing gland, a possibility that has not been explored. We examined this possibility using IHC with an Ab specific for mouse VEGF. As shown in Fig. 5A, VEGF is present in MMTV-Cre, MMTV-Cre;NRP2+/loxP and MMTV-Cre;NRP2loxP/loxP glands as assessed by IHC. The VEGF detected in these glands could have been synthesized by both epithelial and stromal cells, and could affect glandular behavior by autocrine and paracrine mechanisms.
Fig. 5.
VEGF/NRP2 signaling activates FAK, which contributes to branching morphogenesis. (A) Mammary glands from MMTV-Cre, MMTV-Cre;NRP2+/loxP and MMTV-Cre;NRP2loxP/loxP mice were immunostained with an antibody (Ab) specific for mouse VEGF or the appropriate control IgG. Note the intense cytoplasmic staining for VEGF in all of the glands. Scale bar: 10 μm. (B) Mammary glands from 5-week-old MMTV-Cre, MMTV-Cre;NRP2+/loxP and MMTV-Cre;NRP2loxP/loxP mice were immunostained with an Ab specific for p-FAK (Y397) and representative staining is shown. Immunostaining was quantified by scoring the intensity of staining of each gland on a scale of 1-5. Only scores of 4 and 5 were considered positive staining and the data are reported as the intensity of staining in the MMTV-Cre;NRP2+/loxP and MMTV-Cre;NRP2loxP/loxP glands relative to the MMTV-Cre glands. Error bars represent s.d., *P<0.01. Scale bar: 10 μm. (C) NMuMG cells that express the indicated shRNAs were either cultured on tissue culture plates (2D) and stimulated with VEGF or cultured in Matrigel (3D) for 15 days in the presence or absence of VEGF165. Extracts were immunoblotted using a p-FAK or FAK Ab. Note that VEGF165 induces FAK activation in a NRP2-dependent manner in both 2D and 3D culture. (D) NMuMG cells that express one of two independent shRNAs (shNRP-10 and shNRP-76) were transfected with either a constitutively active FAK (K38A) plasmid or vector alone (empty vector). Transfectants were grown in 3D Matrigel cultures for 15 days in the absence of VEGF165. Subsequently, the cells were photographed and branching morphogenesis was quantified. Note that expression of constitutively active FAK restores branching in NRP2-depleted cells. The accompanying immunoblot confirms expression of K38A FAK in the transfectants. Error bars represent s.d., *P<0.01. Scale bars: 50 μm.
The major issue that arises from our data is the mechanism by VEGF/NRP2 signaling promotes branching morphogenesis and mammary gland development. Given the reports that mammary gland branching involves FAK (Nagy et al., 2007; Provenzano et al., 2008; van Miltenburg et al., 2009), we pursued the hypothesis that VEGF/NRP2 signaling activates FAK in mammary epithelial cells and that this pathway accounts for the contribution of VEGF-NRP2 to branching and mammary gland development. This hypothesis is supported by our key observation that the expression of activated FAK (pY397) was diminished significantly in the mammary glands of MMTV-Cre;NRP2loxP/loxP mice compared with control mice (Fig. 5B). Moreover, VEGF induced activation of FAK in both 2D and 3D cultures as assessed by increased FAK phosphorylation (pY397) and this activation was NRP2-dependent (Fig. 5C). To establish the role of FAK in VEGF-NRP2-induced branching more rigorously, we used a constitutively active FAK (K38A) (Cohen and Guan, 2005) to overcome the inhibition of mammary gland branching caused by loss of NRP2. Indeed, expression of K38A FAK in cells expressing NRP2 shRNAs increased branching (Fig. 5D), providing further support for the conclusion that VEGF and NRP2 activate FAK, which contributes to branching morphogenesis.
DISCUSSION
The data reported here demonstrate that NRP2 contributes to proper branching morphogenesis in the developing mouse mammary gland and they indicate that this contribution is mediated by VEGF, an NRP2 ligand that is present in epithelial cells of the developing gland. This hypothesis is supported by our analysis of developing mammary glands in vivo using NRP2-conditional knockout mice as well as in vitro analysis using 3D cultures, which revealed that VEGF induces NRP2-dependent branching morphogenesis of mammary gland epithelial cells. Mechanistically, we demonstrate that VEGF/NRP2 signaling activates FAK, which contributes to branching morphogenesis. Collectively, these findings highlight a novel and potentially important role for NRPs, which were characterized initially for their role in growth cone guidance and neuronal development, in mammary gland morphogenesis.
Branching morphogenesis is a fundamental developmental process that involves the restructuring of epithelial tissues into organized ramified tubular networks (Affolter et al., 2003; Affolter et al., 2009). This process underlies the development of the pubertal mammary gland during which a primitive ductal structure proliferates, branches and extends into the mammary fat pad (Affolter et al., 2003; Ghabrial et al., 2003; Affolter et al., 2009). Although prior studies did not address the potential function of NRPs in mammary gland morphogenesis, such a role was suggested by their expression pattern in the developing mammary gland (Morris et al., 2006) and it is consistent with the ability of these receptors to regulate morphogenetic pathways, especially in neurons (Chen et al., 2005; Vieira et al., 2007). Interestingly, NRP1 has been implicated in branching morphogenesis of renal epithelial cells using an in vitro model system (Karihaloo et al., 2005). In addition, VEGF-A signaling is a major determinant of angiogenic sprouting of endothelial cells (Gerhardt et al., 2003), a form of branching morphogenesis (Affolter et al., 2009).
Our data reveal that the mechanism by which NRP2 promotes branching morphogenesis involves the ability of VEGF-NRP2 to activate FAK. This mechanism is supported by the analysis of mammary gland-specific FAK knockout mice that exhibit delayed ductal outgrowth and branching morphogenesis (Nagy et al., 2007; Provenzano et al., 2008). Interestingly, deletion of FAK in mammary epithelial cells (MECs) results in enhanced Rho kinase-mediated contractility and a failure to respond to receptor-mediated cytoskeletal remodeling (van Miltenburg et al., 2009). Aberrant branching morphogenesis was observed when these FAK-null MECs were transplanted into cleared mammary fat pads. Thus, loss of NRP2 in both the mammary gland and MECs phenocopies some aspects of loss of FAK, and it is reasonable to hypothesize that the ability of FAK to impact cytoskeletal organization and remodeling underlies the contribution of NRP2 to branching morphogenesis. In this direction, VEGF-NRP2 might regulate the function of specific integrins that contribute to FAK activation and are important for branching morphogenesis. This possibility is supported by the report that NRP1 regulates the trafficking and function of the α5β1 integrin in endothelial cells. This mechanism does not involve interaction with other receptors but rather association of the NRP1 cytoplasmic domain with a PDZ-domain binding protein and an associated motor myosin (Valdembri et al., 2009).
Our data provide insight into the relationship between branching morphogenesis in the mammary gland and the EMT. Our seminal finding is that NRP2 has a causal role in both processes, but they are distinct, and branching morphogenesis of the mammary gland does not appear to involve an EMT. This conclusion is supported by time-lapse analysis of ex vivo mammary gland development, which concluded that collective migration drives branching morphogenesis in the mammary gland and that this process occurs in the absence of both an EMT and the formation of cell protrusions at the leading edge (Ewald et al., 2008). Despite these convincing data, the possibility that a localized, transient EMT occurs during branching morphogenesis of the mouse mammary gland and that NRP2 contributes to this EMT cannot be excluded. This possibility is supported by the fact that an EMT is notoriously difficult to capture in vivo (Tarin et al., 2005) and the observation that the expression of genes for transcription factors that repress E-cadherin (cadherin 1 – Mouse Genome Informatics), such as Twist1, Twist2 and Snail is high in TEBs of developing mammary glands (Kouros-Mehr and Werb, 2006). Nonetheless, we conclude from our data that NRP2 has distinct roles in the EMT and branching morphogenesis.
The data presented in this study add to an understanding of the role for specific growth factors in mammary gland development and their relationship to NRP2. Our finding that TGFβ does not induce branching morphogenesis of mammary gland epithelial cells in 3D cultures, although it induces an EMT in 2D, is consistent with previous data demonstrating that TGFβ inhibits ductal extension and branching in the pubertal mammary gland (Roarty and Serra, 2007). This inhibitory effect of TGFβ in vivo is dependent on Wnt5a (Roarty and Serra, 2007), and one possibility to explain the different response of TGFβ in 2D versus 3D cultures is that Wnt5a expression is induced by 3D Matrigel culture. Also, we note that the impact of NRP2 depletion on branching is more pronounced in 3D Matrigel culture than it is in the MMTV-Cre;NRP2loxP/loxP mammary glands. Clearly, stromal factors contribute to the regulation of branching in the mammary gland and such factors are absent in the cell culture system. Nonetheless, the ability of NRP2 to regulate FAK activation is evident in both model systems.
We are intrigued by the observation that VEGF165 is unable to induce an EMT in NMuMG cells in 2D cultures because recent studies have demonstrated the ability of this growth factor to induce an EMT in 2D cultures of breast and prostate carcinoma cells (Wanami et al., 2008; Mak et al., 2010). Nonetheless, VEGF165 is a potent inducer of branching morphogenesis in 3D cultures. The possibility that VEGF functions in the mammary gland in this capacity in vivo is supported by the observation that inactivation of VEGF in the mammary gland severely impedes development of the gland (Rossiter et al., 2007), which was attributed to its role in angiogenesis, but could also involve its interaction with epithelial cells directly. It is possible that semaphorins, especially the NRP2 ligand Sema3f, also contribute to branching morphogenesis and mammary gland development. However, our analysis of a pubertal mammary gland from a Sema3f−/− mouse (provided by Alex Kolodkin, John Hopkins University, MD, USA) revealed no defects in branching or ductal outgrowth (D.B. and A.M.M., unpublished). FGF signaling plays an important role in branching morphogenesis (Affolter et al., 2009), including morphogenesis of the mammary gland (Lu et al., 2006). Interestingly, however, FGF-induced branching of mammary epithelial cells does not appear to involve NRP2, although there is evidence that the NRPs can interact with and modulate the function of specific heparin-binding factor receptors, including FGF receptors (West et al., 2005).
In summary, our results demonstrate that NRP2 contributes to branching morphogenesis in the pubertal mammary gland and implicate VEGF as the NRP2 ligand and FAK as the downstream signaling effector that mediate this contribution. In a broader context, our data support an emerging hypothesis that directional outgrowth and branching morphogenesis in a variety of tissues are influenced by signals that were identified initially for their role in growth cone guidance.
Supplementary Material
Acknowledgements
NIH Grants R01CA89209 (A.M.M.) and T32CA130807 (D.B.) supported this work. We thank Alex Kolodkin for providing the floxed NRP2 mice as well as mammary glands from Sema3f−/− mice, Jun-Lin Guan for providing K38A FAK and Karl Simin (UMASS Medical School) for helpful advice. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.051318/-/DC1
References
- Affolter M., Bellusci S., Itoh N., Shilo B., Thiery J. P., Werb Z. (2003). Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell 4, 11-18 [DOI] [PubMed] [Google Scholar]
- Affolter M., Zeller R., Caussinus E. (2009). Tissue remodelling through branching morphogenesis. Nat. Rev. Mol. Cell Biol. 10, 831-842 [DOI] [PubMed] [Google Scholar]
- Bachelder R. E., Crago A., Chung J., Wendt M. A., Shaw L. M., Robinson G., Mercurio A. M. (2001). Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 61, 5736-5740 [PubMed] [Google Scholar]
- Bachelder R. E., Wendt M. A., Mercurio A. M. (2002). Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 62, 7203-7206 [PubMed] [Google Scholar]
- Bachelder R. E., Lipscomb E. A., Lin X., Wendt M. A., Chadborn N. H., Eickholt B. J., Mercurio A. M. (2003). Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 63, 5230-5233 [PubMed] [Google Scholar]
- Bae D., Lu S., Taglienti C. A., Mercurio A. M. (2008). Metabolic stress induces the lysosomal degradation of neuropilin-1 but not neuropilin-2. J. Biol. Chem. 283, 28074-28080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A., Tessier-Lavigne M., Watts R. J. (2009). Neuropilins in tumor biology. Clin. Cancer Res. 15, 1860-1864 [DOI] [PubMed] [Google Scholar]
- Barr M. P., Byrne A. M., Duffy A. M., Condron C. M., Devocelle M., Harriott P., Bouchier-Hayes D. J., Harmey J. H. (2005). A peptide corresponding to the neuropilin-1-binding site on VEGF(165) induces apoptosis of neuropilin-1-expressing breast tumour cells. Br. J. Cancer 92, 328-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Rivera E., Ran S., Thorpe P., Minna J. D. (2004). Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc. Natl. Acad. Sci. USA 101, 11432-11437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Li M., Chai H., Yang H., Fisher W. E., Yao Q. (2005). Roles of neuropilins in neuronal development, angiogenesis, and cancers. World J. Surg. 29, 271-275 [DOI] [PubMed] [Google Scholar]
- Chen H., Bagri A., Zupicich J. A., Zou Y., Stoeckli E., Pleasure S. J., Lowenstein D. H., Skarnes W. C., Chedotal A., Tessier-Lavigne M. (2000). Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 25, 43-56 [DOI] [PubMed] [Google Scholar]
- Cohen L. A., Guan J. L. (2005). Residues within the first subdomain of the FERM-like domain in focal adhesion kinase are important in its regulation. J. Biol. Chem. 280, 8197-8207 [DOI] [PubMed] [Google Scholar]
- Cowin P., Wysolmerski J. (2010). Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb. Perspect. Biol. 2, a003251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald A. J., Brenot A., Duong M., Chan B. S., Werb Z. (2008). Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., et al. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial A., Luschnig S., Metzstein M. M., Krasnow M. A. (2003). Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 19, 623-647 [DOI] [PubMed] [Google Scholar]
- Giger R. J., Cloutier J. F., Sahay A., Prinjha R. K., Levengood D. V., Moore S. E., Pickering S., Simmons D., Rastan S., Walsh F. S., et al. (2000). Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron 25, 29-41 [DOI] [PubMed] [Google Scholar]
- He Z., Tessier-Lavigne M. (1997). Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90, 739-751 [DOI] [PubMed] [Google Scholar]
- Hu B., Guo P., Bar-Joseph I., Imanishi Y., Jarzynka M. J., Bogler O., Mikkelsen T., Hirose T., Nishikawa R., Cheng S. Y. (2007). Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene 26, 5577-5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karihaloo A., Karumanchi S. A., Cantley W. L., Venkatesha S., Cantley L. G., Kale S. (2005). Vascular endothelial growth factor induces branching morphogenesis/tubulogenesis in renal epithelial cells in a neuropilin-dependent fashion. Mol. Cell. Biol. 25, 7441-7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin A. L., Levengood D. V., Rowe E. G., Tai Y. T., Giger R. J., Ginty D. D. (1997). Neuropilin is a semaphorin III receptor. Cell 90, 753-762 [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H., Werb Z. (2006). Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 235, 3404-3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Sternlicht M. D., Werb Z. (2006). Comparative mechanisms of branching morphogenesis in diverse systems. J. Mammary Gland Biol. Neoplasia 11, 213-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak P., Leav I., Pursell B., Bae D., Yang X., Taglienti C. A., Gouvin L. M., Sharma V. M., Mercurio A. M. (2010). ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 17, 319-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita A., Gotze T., Korc M. (2007). Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 67, 10309-10316 [DOI] [PubMed] [Google Scholar]
- Morris J. S., Stein T., Pringle M. A., Davies C. R., Weber-Hall S., Ferrier R. K., Bell A. K., Heath V. J., Gusterson B. A. (2006). Involvement of axonal guidance proteins and their signaling partners in the developing mouse mammary gland. J. Cell. Physiol. 206, 16-24 [DOI] [PubMed] [Google Scholar]
- Nagy T., Wei H., Shen T. L., Peng X., Liang C. C., Gan B., Guan J. L. (2007). Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J. Biol. Chem. 282, 31766-31776 [DOI] [PubMed] [Google Scholar]
- Neufeld G., Kessler O., Herzog Y. (2002). The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv. Exp. Med. Biol. 515, 81-90 [DOI] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Beggs H. E., Keely P. J. (2008). Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am. J. Pathol. 173, 1551-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roarty K., Serra R. (2007). Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development 134, 3929-3939 [DOI] [PubMed] [Google Scholar]
- Rossiter H., Barresi C., Ghannadan M., Gruber F., Mildner M., Fodinger D., Tschachler E. (2007). Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J. 21, 3994-4004 [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735-745 [DOI] [PubMed] [Google Scholar]
- Sulpice E., Plouet J., Berge M., Allanic D., Tobelem G., Merkulova-Rainon T. (2008). Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 111, 2036-2045 [DOI] [PubMed] [Google Scholar]
- Tarin D., Thompson E. W., Newgreen D. F. (2005). The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 65, 5996-6001 [DOI] [PubMed] [Google Scholar]
- Timoshenko A. V., Rastogi S., Lala P. K. (2007). Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br. J. Cancer 97, 1090-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniewicz K. A., Fernig D. G. (2008). Neuropilins: a versatile partner of extracellular molecules that regulate development and disease. Front. Biosci. 13, 4339-4360 [DOI] [PubMed] [Google Scholar]
- Valdembri D., Caswell P. T., Anderson K. I., Schwarz J. P., Konig I., Astanina E., Caccavari F., Norman J. C., Humphries M. J., Bussolino F., et al. (2009). Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 7, e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Miltenburg M. H., Lalai R., de Bont H., van Waaij E., Beggs H., Danen E. H., van de Water B. (2009). Complete focal adhesion kinase deficiency in the mammary gland causes ductal dilation and aberrant branching morphogenesis through defects in Rho kinase-dependent cell contractility. FASEB J. 23, 3482-3493 [DOI] [PubMed] [Google Scholar]
- Vieira J. M., Schwarz Q., Ruhrberg C. (2007). Selective requirements for NRP1 ligands during neurovascular patterning. Development 134, 1833-1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K. U., Wall R. J., St-Onge L., Gruss P., Wynshaw-Boris A., Garrett L., Li M., Furth P. A., Hennighausen L. (1997). Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25, 4323-4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A., Rodriguez I., Mombaerts P. (2002). Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J. Neurosci. 22, 4025-4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanami L. S., Chen H. Y., Peiro S., Garcia de Herreros A., Bachelder R. E. (2008). Vascular endothelial growth factor-A stimulates Snail expression in breast tumor cells: implications for tumor progression. Exp. Cell Res. 314, 2448-2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D. C., Rees C. G., Duchesne L., Patey S. J., Terry C. J., Turnbull J. E., Delehedde M., Heegaard C. W., Allain F., Vanpouille C., et al. (2005). Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J. Biol. Chem. 280, 13457-13464 [DOI] [PubMed] [Google Scholar]
- Yang X., Pursell B., Lu S., Chang T. K., Mercurio A. M. (2009). Regulation of beta 4-integrin expression by epigenetic modifications in the mammary gland and during the epithelial-to-mesenchymal transition. J. Cell Sci. 122, 2473-2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka H., Kodama R., Tsujimoto M., Yoshidome K., Akamatsu H., Nakahara M., Inagaki M., Sanke T., Nakamura Y. (2009). Neuropilin-2 expression in breast cancer: correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer 9, 220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.