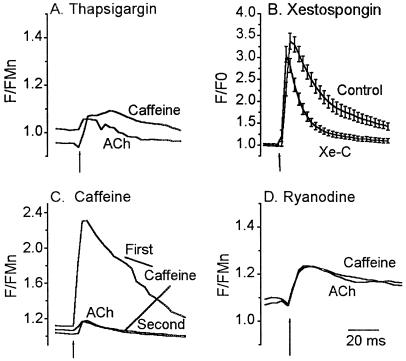
Figure 4.
αBgt-AChR-mediated calcium response is primarily caused by CICR. (A) Depletion of all intracellular calcium stores by TG abolished response to ACh. Cells were incubated in 1 μM TG for 30 min, washed, and then challenged with 20 mM caffeine (Caffeine) to deplete any residual store calcium. Subsequent application of ACh lead to a small increase in calcium, on average about 2% of control, indicating that most of the rise in cytosolic calcium is due to release from intracellular stores. Data are the mean response from 28 cells. (B) InsP3 stores contribute to ACh-induced calcium transient. Untreated astrocytes (Control) or astrocytes treated with 20 μM Xe-C for 30 min (Xe-C) were challenged with 100 μM ACh for 2 s. ACh elicited a large calcium transient in Xe-C-treated cells that decayed faster than that obtained from untreated cells. Data are the mean ± SEM from 38 cells (Xe-C) and 42 cells (Control). (C) Caffeine stores are necessary for αBgt-AChR-mediated calcium response. Application of 20 mM caffeine for 2 s depleted calcium stores. Application of ACh 90 s later produced a small response as did a second application of 20 mM caffeine, suggesting that αBgt-AChRs and caffeine share a common pool of intracellular calcium. Data are the mean transient from 38 cells. (D) ACh response can be blocked by ryanodine. Cells were incubated with 100 μM ryanodine for 30 min to block ryanodine receptors. Cells were challenged with 20 mM caffeine for 2 s, followed 5–7 min later by 100 μM ACh for 2 s. Responses to both ACh (lower trace) and caffeine (upper trace) were blocked, suggesting that the rise in cytosolic calcium resulting from activation of αBgt-AChRs is indeed amplified by CICR. Data are the mean response from 40 cells. Basal F/FMn levels after incubation with TG and ryanodine were 1.02 ± 0.04 and 1.1 ± 0.05, respectively. All responses were in the presence of 500 nM atropine.