Abstract
Background and Aims
In seeds with deep simple epicotyl morphophysiological dormancy, warm and cold stratification are required to break dormancy of the radicle and shoot, respectively. Although the shoot remains inside the seed all winter, little is known about its growth and morphological development prior to emergence in spring. The aims of the present study were to determine the temperature requirements for radicle and shoot emergence in seeds of Viburnum betulifolium and V. parvifolium and to monitor growth of the epicotyl, plumule and cotyledons in root-emerged seeds.
Methods
Fresh and pre-treated seeds of V. betulifolium and V. parvifolium were incubated under various temperature regimes and monitored for radicle and shoot emergence. Growth of the epicotyl and cotyledons at different stages was observed with dissecting and scanning electron microscopes.
Key Results
The optimum temperature for radicle emergence of seeds of both species, either kept continuously at a single regime or exposed to a sequence of regimes, was 20/10 °C. GA3 had no effect on radicle emergence. Cold stratification (5 °C) was required for shoot emergence. The shoot apical meristem in fresh seeds did not form a bulge until the embryo had grown to the critical length for radicle emergence. After radicle emergence, the epicotyl–plumule and cotyledons grew slowly at 5 and 20/10 °C, and the first pair of true leaves was initiated. However, the shoot emerged only from seeds that received cold stratification.
Conclusions
Seeds of V. betulifolium and V. parvifolium have deep simple epicotyl morphophysiological dormancy, C1bB (root)–C3 (epicotyl). Warm stratification was required to break the first part of physiological dormancy (PD), thereby allowing embryo growth and subsequently radicle emergence. Although cold stratification was not required for differentiation of the epicotyl–plumule, it was required to break the second part of PD, thereby allowing the shoot to emerge in spring.
Keywords: Epicotyl morphophysiological dormancy, epicotyl–plumule growth, radicle emergence, seed germination, shoot growth, Viburnum
INTRODUCTION
A seed that does not germinate within about 30 d under conditions suitable for the non-dormant seed to do so is said to be dormant (Baskin and Baskin, 1998). The challenge for the seed ecologist is to determine why there is a delay in germination and why seeds eventually germinate at some specific season(s). Information on the environmental conditions required to promote germination provides valuable insight into what controls timing of germination in the field and ultimately into how the seed/seedling stage is adapted to a particular habitat (Baskin and Baskin, 1998).
As dormant seeds gain the ability to germinate at some particular time(s) during the year, many physiological and/or morphological changes may occur in them. Thus, to understand why there is a delay in germination and how dormancy is broken, information is needed on the various changes that occur in the seeds and how they are correlated with the annual climatic cycle in the habitat. A case in point is seeds with an underdeveloped embryo that also have a physiological inhibiting mechanism of germination, i.e. morphophysiological dormancy (MPD) (Nikolaeva, 1969). That is, the embryo must grow and develop morphologically and the physiological limitations must be overcome before the seed can germinate.
There are nine different kinds (levels) of MPD, based on temperature requirements for embryo growth, breaking of physiological dormancy (PD) and emergence of the radicle and shoot, and on the response of seeds to GA3 (Nikolaeva, 1969; Baskin and Baskin, 1998; Baskin et al., 2008). One level of MPD that has received considerable research attention is deep simple epicotyl MPD, in which warm stratification is required to break PD of the radicle and cold stratification is required to break PD of the shoot (epicotyl). In these seeds, the radicle emerges in autumn and the shoot in spring (Baskin and Baskin, 1998).
It is known that considerable embryo growth occurs prior to radicle emergence in seeds with deep simple epicotyl MPD (e.g. Kondo et al., 2002, 2004; Hidayati et al., 2005; Karlsson et al., 2005). However, although the shoot remains inside seeds with deep simple epicotyl MPD all winter, very little is known about the changes that occur in the epicotyl during exposure to chilling (0–10 °C) temperature, especially its growth and morphological development. The only previous study we are aware of on epicotyl differentiation and development in seeds with epicotyl dormancy is the one by Barton and Chandler (1957) on Paeonia suffructicosa. They found that although the epicotyl differentiated in untreated seedlings (i.e. root-emerged seeds), a shoot was not produced, i.e. dormancy of the epicotyl was not broken. On the other hand, seedlings treated with GA3 or given an 8 week period at 5 °C produced green shoots.
Our study primarily addresses shoot development after radicle emergence in seeds with deep simple epicotyl MPD and was conducted using seeds of Viburnum betulifolium and V. parvifolium from high elevations in Taiwan. Since spring is the natural time for shoot emergence, we hypothesized that (a) the shoot would be well developed in spring and (b) low winter temperatures (cold stratification) would be required for this development. Thus, this study had two objectives: (1) to determine the dormancy breaking and germination requirements of V. betulifolium and V. parvifolium; and (2) to investigate morphological changes that occur in the shoot prior to its emergence, thereby evaluating the two hypotheses related to development.
Viburnum is a member of the Adoxaceae, which is one of six families in the evolutionarily highly advanced order Dipsacales with MPD (Baskin et al., 2006). Further, seeds of many species in the genus Viburnum have a linear differentiated (root and cotyledons present), underdeveloped (small) embryo, and the embryo has PD. Thus, the seeds have MPD, and many species of Viburnum have deep simple epicotyl MPD (Baskin et al., 2009a).
MATERIALS AND METHODS
Study organisms
Twelve of the 15 species of Viburnum in Taiwan are distributed in natural forests at elevations >1000 m; all of them are shrubs. The two highest elevation species of Viburnum in Taiwan, and the subjects of this study, are V. betulifolium Batal. (2300–3100 m) and V. parvifolium Hayata (2500–3300 m). The former species occurs in China and Taiwan, and the latter is endemic to Taiwan and is becoming rare (Yang and Chiu, 1998). The red fruits of both V. betulifolium and V. parvifolium mature in November and are dispersed as the leaves fall in December.
Fruit harvest, seed handling and definition of tissues in the embryo
Mature red to dark-red fruits of V. betulifolium were collected from openings in a mixed conifer–broadleaved forest in Hsiaofengkou (24°10′N, 121°16′E), central Taiwan, at an elevation of 3000 m a.s.l. in November 2004, 2005 and 2007. Mature red to dark-red fruits of V. parvifolium were collected from openings in a broadleaved forest in Tayuling (24°11′N, 121°17′E), central Taiwan, at an elevation of 2600 m a.s.l. in November 2005 and 2007. Seeds were extracted from fruits by removing the pulp (exocarp + mesocarp) by hand in water. The cleaned seeds (with endocarp) that sank in water were air-dried on newspapers for 2 d at ambient laboratory temperature (approx. 25 °C), after which they were stored temporarily in a sealable polyethylene bag at 5 °C for 2 weeks.
The fruit of both species is a one-seeded drupe consisting of a thin exocarp, fleshy mesocarp and a hard endocarp that is united with the seed coat. The germination unit (true seed plus endocarp, hereafter seed) consists of the endocarp, seed coat and a small linear-shaped embryo surrounded by a large amount of endosperm. Mean seed size (n = 20) of V. betulifolium was 5·40 ± 0·53 mm long, 4·14 ± 0·29 mm wide and 1·70 ± 0·23 mm thick, and for V. parvifolium 6·32 ± 0·59 mm long, 4·56 ± 0·21 mm wide and 1·88 ± 0·13 mm thick. There were about 22 350 and 18 050 seeds, respectively, per litre.
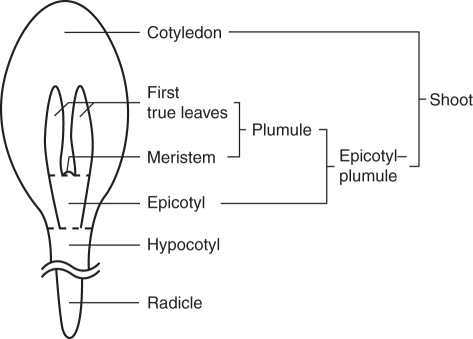
The small embryo of a freshly matured Viburnum seed consists of the embryonic axis (radicle and hypocotyl) and cotyledons. The epicotyl-plumule is the embryonic shoot above the attachment of the cotyledons but is not visible in the mature seed until the embryo grows to some extent inside the seed. The first true leaves with the meristem are visible after radicle emergence (one of the topics in the paper; Fig. 1). Here, we define the plumule to include the first true leaves plus meristem and the shoot to include the epicotyl–plumule and cotyledons (Fig. 1).
Fig 1.
Diagram showing the location of the radicle, hypocotyl, epicotyl, meristem, first true leaves, plumule, cotyledons and shoot in Viburnum embryo. One of the cotyledons has been removed to show the epicotyl–plumule. Not drawn to scale.
Imbibition test
The rate of water uptake was monitored in seeds of the two Viburnum species collected in 2005. Each of 20 non-scarified and scarified (cut at a point away from micropylar end) seeds was weighed individually to the nearest 0·1 mg at time 0 and at various times after incubation for 7 d at ambient laboratory temperature on Whatman No. 1 filter paper in Petri dishes moistened with distilled water. Thus, seed mass was determined at about 8 h intervals for the first 24 h and then once every 24 h for the next 6 d. Percentage water uptake (%Ws) was calculated as %Ws = [(Wi – Wd)/Wd] × 100, where Ws = increase in seed mass, Wi = seed mass after water uptake (imbibition) for a given period of time and Wd = initial seed mass. The initial water content (fresh weight basis) of V. betulifolium and V. parvifolium was determined by oven drying for 17 h at 103 °C (International Seed Testing Association, 1999).
Effect of temperature regimes on radicle emergence and embryo growth
The air-dried seeds of V. betulifolium collected in 2004 were sent by air mail to the University of Kentucky, USA, and studies were initiated within 3 weeks of seed collection. The seeds were incubated on sand moistened with distilled water in 9 cm diameter plastic Petri dishes. Seeds were incubated at (12/12 h) alternating temperature regimes of 25/15, 20/10 and 15/6 °C and at a constant temperature of 5 °C. The daily photoperiod was 14 h. In the alternating temperature regimes, lights came on 1 h before the beginning of the high temperature period and went off 1 h after the beginning of the low temperature period. Photon irradiance was about 40 µmol m−2 s−1 of cool white fluorescent light, 400–700 nm. At each temperature, three replicates of 50 seeds were used. Seeds were monitored at 2 week intervals for 104 weeks, and those with a radicle ≥1 mm were recorded as germinated and were removed from the dish. Embryo growth was monitored for 38 weeks in the V. betulifolium seeds incubated at 25/15, 20/10, 15/6 and 5 °C. Embryos were dissected from seeds using a razor blade, and the length of 15 embryos was measured at each monitoring period under a dissecting microscope equipped with a calibrated micrometer. To determine the critical embryo length, i.e. the length of the embryo including radicle–hypocotyl and cotyledons just before the radicle emerges, seeds for which the seed coat had split but the radicle had not emerged were excised and measured.
Studies on seeds of V. betulifolium and V. parvifolium collected in 2005 were carried out in Taiwan. They were mixed with moist sphagnum moss cut into small pieces (water content of the sphagnum was about 400 % of its dry mass) and sealed inside transparent polyethylene bags (0·04 mm in thickness). The moist sphagnum provided a good germination medium and prevented the spread of mould because it contains the fungi Trichoderma and actinomycetes that are antagonistic to micro-organisms (Wang et al., 1998). The seeds were covered with ≤3 mm of sphagnum moss. Seeds were incubated at 30/20, 25/15, 20/10, 15/6 and 5 °C. Daily photoperiod (cool white fluorescent light, 80–100 µmol m−2 s−1, 400–700 nm; hereafter light) during incubation was 12 h at the higher temperature; the 5 °C regime was in continuous darkness in a cold room. Due to the coarse nature of the sphagnum, most seeds received some light, but at any given point in time a few may have been in darkness. However, at weekly intervals the contents of each bag were poured out on a table to facilitate examination of seeds for germination. After germination was monitored, seeds and sphagnum were returned to the bag, resulting in a re-shuffling of seeds with regard to their position in the sphagnum and thus the light they received. Consequently, all seeds were in light part (or all) of the time the lights were on in the incubator. Each treatment consisted of three replicates of 50 seeds. Most seeds incubated on/in sphagnum moss remained healthy and viable at all temperatures during the ≤104 weeks of incubation, and those with a radicle ≥2 mm were recorded as germinated and were removed from the bag. Only a few of the seeds rotted, and they were picked out of the germination medium during the investigation. Means and standard errors (s.e.) were calculated for each time measurement, and results are expressed as germination percentage.
Effect of cold to warm and warm to cold temperature sequences on radicle emergence, shoot emergence and embryo growth
Since seeds of V. betulifolium and V. parvifolium are dispersed in December, the first temperature treatment they would receive in the field is cold stratification. Moist cold stratification has been used to overcome dormancy and promote germination in dormant seeds of many species (Bewley and Black, 1994; Baskin and Baskin, 1998). However, dormancy break may require only cold stratification, only warm stratification, warm followed by cold stratification or cold followed by warm stratification (Baskin and Baskin, 1998). Thus, the purpose of this experiment was to determine if cold, warm or warm plus cold stratification treatments were required for dormancy break and emergence of root and shoot (Baskin and Baskin, 2003). Seeds of V. betulifolium and V. parvifolium were subjected to temperature sequences simulating natural warm to cold and cold to warm stratification in the field. Seeds of these two species collected in 2005 were mixed with moist sphagnum moss, sealed inside polyethylene bags and incubated in light as described above. Three replications of 50 seeds each, plus extra bags of seeds for the embryo growth study, were subjected to the following two temperature sequences: (1) beginning with warm temperature, 25/15 °C for 12 weeks → 20/10 °C for 8 weeks → 15/6 °C for 4 weeks → 5 °C for 8 weeks, then moving the seeds back to 15/6, 20/10, 25/15 °C and continuing the sequence if they had not germinated; and (2) beginning with cold temperature, 5 °C for 8 weeks → 15/6 °C for 4 weeks → 20/10 °C for 8 weeks → 25/15 °C for 12 weeks → 20/10 °C for 8 weeks → 15/6 °C for 4 weeks → 5 °C for 8 weeks, then moving the seeds back to 15/6, 20/10, 25/15 °C and continuing the sequence if they had not germinated. Most seeds incubated on sphagnum moss remained healthy and viable at all temperatures during the incubation, and those with a radicle ≥2 mm were recorded as germinated and removed from the bag. Only a few of the seeds rotted, and they were picked out of the germination medium during the investigation. Means and s.e. were calculated for each time measurement, and results are expressed as germination percentage. In temperature sequence number (1), ten seeds were dissected biweekly using razor blades, and the length of the embryos (including radicle–hypocotyl and cotyledons) and of seeds was measured under a dissecting microscope equipped with a calibrated micrometer. The radicle-emerged seeds (length of radicle 2–5 mm) were incubated continuously at the sequence of temperatures and monitored for shoot emergence.
Effect of temperature on shoot emergence of radicle-emerged seeds
The radicle-emerged seeds (length of radicle 2–5 mm) from the 20/10 °C incubation were mixed with moist sphagnum moss and sealed inside polyethylene bags; each treatment consisted of three replicates of ten radicle-emerged seeds. Seeds of both species were collected in 2005. The radicle-emerged seeds were then incubated in light at 30/20, 25/15, 20/10, 15/6 and 5 °C, and shoot emergence was recorded weekly for 34 weeks. After 34 weeks of incubation, the radicle-emerged seeds at the above temperatures gradually became rotten.
Effect of GA3 on radicle emergence
The purpose of this experiment was to determine if GA3 would break radicle dormancy in seeds of the two Viburnum species. Fresh, intact seeds of V. betulifolium and V. parvifolium collected in 2007 were soaked in 0 (water control) and in solutions of 100, 500, 1000 or 2000 ppm GA3 (as K-GA3; 95 % purity, Sigma) for 48 h at room temperature prior to being tested for germination. Treated seeds were mixed with moist sphagnum moss and incubated in light at 25/15 and 20/10 °C. Each treatment consisted of three replications of 50 seeds. Most seeds incubated on sphagnum moss remained healthy and viable both at 25/15 and 20/10 °C during incubation, and those with a radicle ≥2 mm were recorded as germinated and removed from the bag. Means and s.e. were calculated for each time measurement, and results are expressed as germination percentage.
Effect of temperature on growth of cotyledons and epicotyl–plumule inside radicle-emerged seeds
Growth of cotyledons and of the epicotyl–plumule inside radicle-emerged seeds (length of radicle 2–5 mm) was monitored at 5 and at 20/10 °C, which approximate winter and spring habitat temperatures, respectively. Preliminary tests showed that shoots of both species would emerge at 5 °C but not at 30/20, 25/15 or 20/10 °C. Seeds of the two species were collected in 2007, and >1000 seeds of each species were incubated at 20/10 °C for radicle emergence. More than 500 radicle-emerged seeds of each species were mixed with sphagnum moss and incubated either at 5 °C in darkness, 20/10 °C in light or 5 °C in darkness → 20/10 °C in light. At various time intervals, the length of the cotyledons and of the epicotyl–plumule was determined inside radicle-emerged seeds of V. betulifolium incubated in light at 20/10 °C for 24 weeks and in darkness at 5 °C for 20 weeks and of radicle-emerged seeds of V. parvifolium incubated in light at 20/10 °C for 14 weeks and in darkness at 5 °C for 16 weeks. In addition, radicle-emerged seeds with sphagnum moss kept at 5 °C in darkness for 12 weeks were moved to 20/10 °C in light for 11 and 2 weeks for V. betulifolium and V. parvifolium, respectively; thus, in these temperature treatments, cotyledon and epicotyl–plumule lengths were measured for a total of 23 (12 + 11) and 14 (12 + 2) weeks, respectively. The lengths of 13–20 cotyledons and epicotyl–plumules were measured at 4-week intervals. For observations on the cotyledons and the epicotyl–plumule, the endocarp and endosperm were removed using a razor blade, and one of the cotyledons was also excised to reveal the epicotyl–plumule. The cotyledon and epicotyl–plumule were observed under a WILD M3Z dissecting microscope (Leica) and photographed with a COOLPIX 4500 digital camera (Nikon). The length of cotyledons was measured with a digital caliper (±0·005 mm), while that of epicotyl–plumules was determined from the micrographs using Image J (NIH Image).
Observations on epicotyl–plumule development and cotyledon growth
To document development of the first true leaves, the shoot apical meristem and/or tissues developing from the meristem were examined in fresh seeds, seeds with their coat split and inside seeds with the radicle emerged to a length of 1 mm. Seeds of the two species collected in 2007 were mixed with sphagnum moss and incubated at 20/10 °C in light. Embryos were separated from the seeds and fixed with 2·5 % glutaraldehyde (in 1·0 % sodium phosphate buffer) and then with 1·0 % osmium tetraoxide (in 1·0 % sodium phosphate buffer). Embryos subsequently were dehydrated in an ethanol series and transferred into pure acetone for critical point dehydration (Hitachi Critical Point Dryer, HCP-2, Japan). One of the cotyledons was removed from each embryo to reveal the apical meristem or first true leaves, and then the embryos were coated with IB-2 Ion Coater (Eiko Engineering Co., Japan) and photographed by scanning electron microscopy with an FEI Inspect S (FEI Company, USA). Cotyledons and epicotyl–plumules were photographed under a WILD M3Z dissecting microscope (Leica).
Statistical analysis
Germination data based on number of seeds in a treatment were converted to percentages, and means (three replications) and s.e. were calculated. Statistical analysis of cotyledon and epicotyl growth was carried out using the GLM procedure of SAS, and means were compared by least significant difference (SAS Institute Inc., Cary, NC, USA). Differences in cotyledon or epicotyl (including plumule) length at 5 °C vs. 20/10 °C for each period of time were compared by Student's t-test.
RESULTS
Imbibition
The initial water content of V. betulifolium and V. parvifolium seeds was 32·0 and 19·3 %, respectively. During imbibition, the mass of intact non-scarified V. betulifolium and V. parvifolium seeds increased by 14·3 and 31·8 %, respectively, in 1 d, with an additional increase of 1·8 and 10·6 %, respectively, after 7 d (data not shown). Thus, the moisture content of fully hydrated V. betulifolium and V. parvifolium seeds was 46·3 and 51·1 % (dry weight basis), respectively.
Effect of temperature regimes on radicle emergence and embryo growth
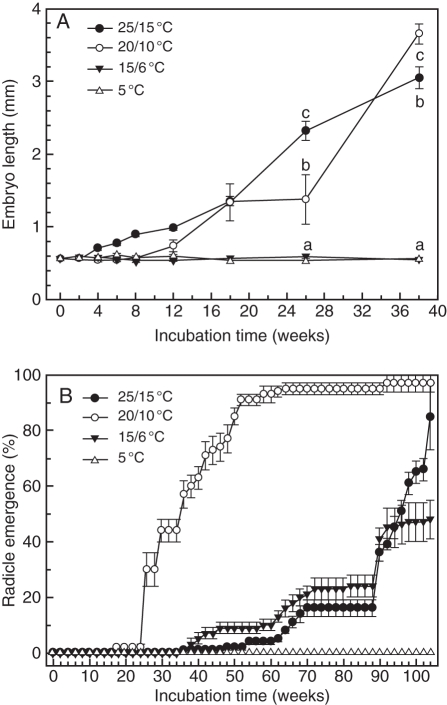
Mean (± s.e.) length of embryos in fresh seeds of V. betulifolium (n = 15) collected in 2004 was 0·564 ± 0·023 mm, and the critical embryo length, including radicle–hypocotyl and cotyledons, just before the radicle emerges was 3·65 ± 0·14 mm. Thus, embryo length increased 650 % before radicle emergence (Fig. 2). The embryo length/seed length (E:S) ratio of fresh seeds was 0·104. Embryo growth in 2004-collected seeds was initiated first at 25/15 °C, and at 26 weeks the embryo length in seeds at 25/15 °C was significantly greater than that in seeds at 20/10 °C (Fig. 3A). However, after 38 weeks embryos in seeds at 20/10 °C were significantly longer than those in seeds at 25/15 °C. The optimum temperature for germination (radicle emergence) of 2004-collected seeds was 20/10 °C, and 50 % of the seeds at this temperature had germinated (emerged radicle) at week 36. Seeds at 25/15 °C reached 50 % germination at week 96, and after 104 weeks only 48 % of seeds at 15/6 °C and none at 5 °C had germinated (Fig. 3B).
Fig. 2.
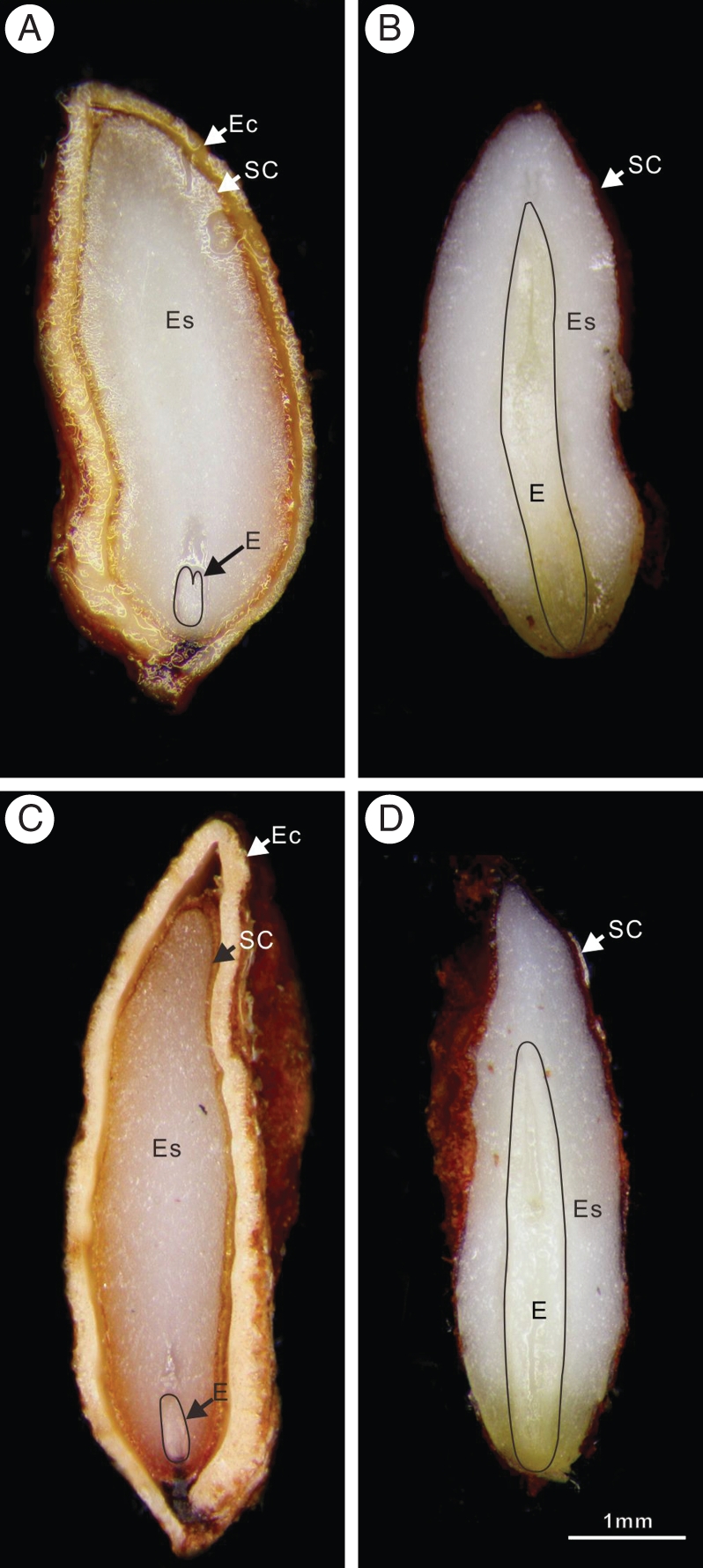
Longitudinal sections of Viburnum betulifolium (A, B) and Viburnum parvifolium (C, D) seeds showing a small linear embryo (A, C) at the time of seed maturity and a fully grown embryo at the time when the seed coat splits (B, D). Endocarp was removed in (B) and (D). Seeds were collected in 2007. Abbreviations: E, embryo; Ec, endocarp; Es, endosperm; SC, seed coat.
Fig. 3.
Effect of temperature on embryo growth (top) and radicle emergence (bottom) of Viburnum betulifolium seeds collected in 2004 and incubated for 104 weeks under various temperature regimes. Points with a different letter are significantly different at that incubation time (l.s.d., P = 0·05). Vertical bars are ± s.e.
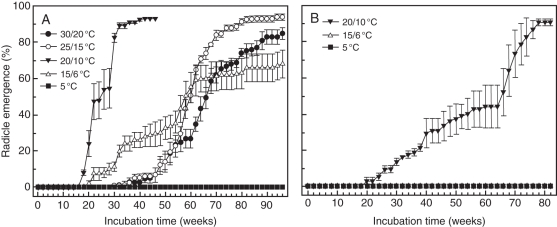
The optimum germination temperature of V. betulifolium seeds collected in 2005 was 20/10 °C, and 50 % of the seeds had germinated at week 24. Fifty per cent of the seeds at 15/6, 25/15 and 30/20 °C had germinated at week 58, 60 and 66, respectively (Fig. 4A).
Fig. 4.
Effect of temperature on radicle emergence in Viburnum betulifolium (A) and Viburnum parvifolium (B) seeds collected in 2005 and incubated under various temperature regimes. Data are not available for V. parvifolium at 30/20 or 25/15 °C. Vertical bars are ± s.e.
Mean (±s.e.) length of embryos in fresh seeds of V. parvifolium (n = 15) collected in 2005 was 0·648± 0·037 mm, and the critical embryo length, including radicle–hypocotyl and cotyledons, just before the radicle emerges was 4·15 ± 0·23 mm. Thus, embryo length increased 640 % before radicle emergence. The E:S ratio was 0·102. The optimum temperature for germination of V. parvifolium collected in 2005 was 20/10 °C, but seeds did not reach 50 % germination until week 66 (Fig. 4B). No seeds germinated at 15/6 or 5 °C.
Effect of cold to warm and warm to cold temperature sequences on radicle emergence, epicotyl emergence and embryo growth
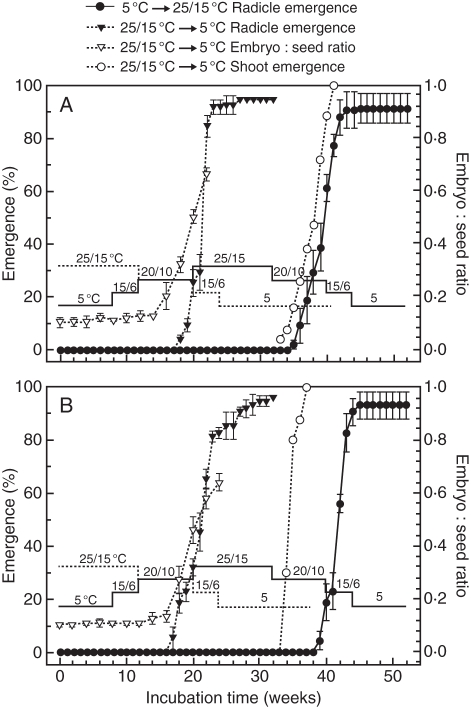
For seeds of V. betulifolium incubated in the temperature sequence beginning at 25/15 °C, radicle emergence began near the end of the 20/10 °C regime and increased rapidly to >90 % in the early part of the 15/6 °C regime. Meanwhile, embryos grew and the E:S ratio increased to 0·66 while seeds were incubated at the 20/10 °C regime (Figs 1 and 5A). Shoots emerged from all of the seeds during the 16 week period of incubation at 5 °C. In the temperature sequence beginning at 5 °C, no seeds germinated until they were moved to 20/10 °C the second time. At this temperature regime, radicle emergence percentage increased rapidly to 62 %, and it reached 91 % shortly after seeds were transferred to 15/6 °C (Fig. 5A).
Fig. 5.
Cumulative radicle emergence in seeds of Viburnum betulifolium (A) and Viburnum parvifolium (B) incubated under a temperature sequence beginning at 5 °C or at 25/15 °C. Embryo length and shoot emergence were measured during the temperature sequence beginning at 25/15 °C. Seeds of V. betulifolium and V. parvifolium were collected in 2005. Error bars are ± s.e.
For seeds of V. parvifolium incubated in the temperature sequence beginning at 25/15 °C, radicle emergence began at 20/10 °C, increased to 83 % at 15/6 °C and then to 96 % at 5 °C. The E:S ratio increased to 0·64 in the 20/10 °C and 15/6 °C regimes. Shoots emerged from all of the seeds during the 8 week period of incubation at 5 °C (Figs 1 and 5B). In the temperature sequence beginning at 5 °C, no seeds germinated until they were moved to 20/10 °C the second time. Radicle emergence percentage reached 19 % at 20/10 °C, and then it increased to 94 % in the 15/6 °C regime (Fig. 5B).
Effect of temperature on shoot emergence of radicle-emerged seeds
Shoot emergence occurred at 5 °C for seeds of both V. betulifolium and V. parvifolium. However, only 15·5 % of the shoots of V. betulifolium and 0 % of those of V. parvifolium emerged at 15/6 °C during 32 weeks incubation. No shoots of either species emerged at 20/10, 25/15 or 30/20 °C (Table 1). Radicle-emerged seeds of both species died before epicotyls emerged during >32 weeks incubation at 20/10, 25/15 or 30/20 °C.
Table 1.
Effect of temperature on shoot emergence in radicle-emerged seeds of Viburnum betulifolium and V. parvifolium
| Species | Temperature (°C) | Incubation time (weeks) | Shoot emergence (%) |
|---|---|---|---|
| Viburnum betulifolium | 30/20 | 32 | 0 |
| 25/15 | 32 | 0 | |
| 20/10 | 32 | 0 | |
| 15/6 | 32 | 15·5 | |
| 5 | 17 | 100 | |
| Viburnum parvifolium | 30/20 | 32 | 0 |
| 25/15 | 32 | 0 | |
| 20/10 | 32 | 0 | |
| 15/6 | 32 | 0 | |
| 5 | 17 | 100 |
Effect of GA3 on radicle emergence
GA3 did not increase radicle emergence in fresh seeds of either V. betulifolium or V. parvifolium incubated at 20/10 or 25/15 °C. When seeds of V. betulifolium were pre-treated with 0 (control), 100, 500, 1000 and 2000 ppm GA3 and re-incubated at 20/10 °C for 20 weeks, 16·0 ± 5·9, 14·7 ± 4·1, 15·3 ± 5·0, 18·0 ± 4·3 and 14·7 ± 5·0 % of them germinated, respectively. After incubation for 30 weeks, germination had increased, and 61·3 ± 6·6, 56·7 ± 3·4, 63·3 ± 3·4, 57·3 ± 0·9 and 65·3 ± 4·7 % of them germinated, respectively. When seeds of V. parvifolium were pre-treated with 0 (control), 100, 500, 1000 and 2000 ppm GA3 and re-incubated at 20/10 °C for 30 weeks, 8·0 ± 3·3, 5·3 ± 1·9, 9·3 ± 0·9, 9·3 ± 2·9 and 8·0 ± 2·8 % of them germinated, respectively. Again, germination had increased after incubation for 50 weeks to 32·3 ± 8·2, 34·0 ± 7·1, 30·7 ± 7·5, 35·3 ± 6·8 and 36·7 ± 8·6 %, respectively. No seeds of V. betulifolium and V. parvifolium pre-treated with GA3 and re-incubated at 25/15 °C for 30 weeks germinated. After incubation for 50 weeks, seeds of V. betulifolium germinated to <10 %, and no seeds of V. parvifolium germinated.
Effect of temperature on growth of cotyledons and epicotyl–plumule inside radicle-emerged seeds
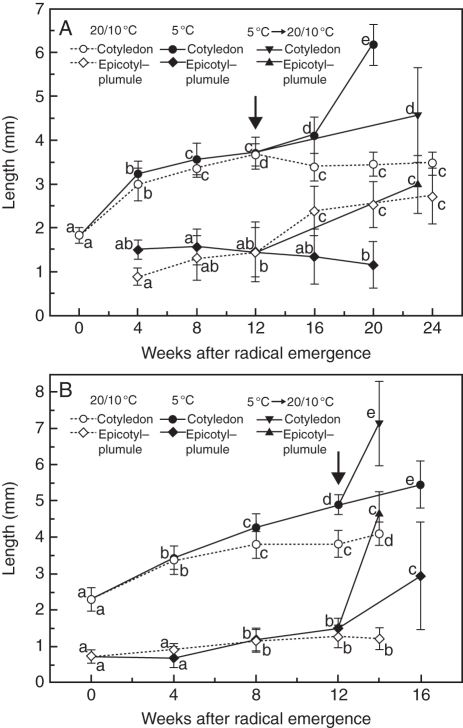
The length of the cotyledons increased from 1·8 to 3·6 mm inside radicle-emerged seeds of V. betulifolium during 12 weeks incubation at 20/10 °C, after which little growth occurred. The epicotyl–plumule increased from 0·9 to 1·4 mm in length inside radicle-emerged seeds during the first 12 weeks of incubation at 20/10 °C and then to 2·7 mm in the following 12 week period under this temperature regime (Fig. 6A). The length of the cotyledons increased from 3·0 to 4·1 mm during 16 weeks incubation at 5 °C, but thereafter the cotyledons grew rapidly to 6·2 mm and emerged from seeds within 20 weeks (Fig. 6A). Cotyledon length in seeds incubated for 16 or 20 weeks at 5 °C was significantly higher (t-test, P < 0·001) than that in seeds incubated at 20/10 °C, but epicotyl–plumule length in seeds incubated for 4, 16 or 20 weeks at 20/10 °C was significantly higher (t-test, P < 0·001) than that in seeds incubated at 5 °C.
Fig. 6.
Growth curves for the cotyledon and epicotyl–plumule inside radicle-emerged seeds of Viburnum betulifolium incubated at 20/10 or 5 °C for 20–24 weeks (A) and of V. parvifolium seeds incubated at 20/10 or 5 °C for 14–16 weeks (B). The radicle-emerged seeds of V. betulifolium and V. parvifolium were at 5 °C for 12 weeks and then were moved (arrow) to 20/10 °C for another 11 and 2 weeks, respectively. Means (n = 13–20) for each curve with the same letter do not differ significantly (l.s.d., P = 0·05). Seeds of V. betulifolium and V. parvifolium were collected in 2007. Vertical bars are ±1 s.e.
The length of cotyledons inside radicle-emerged seeds of V. parvifolium increased slightly during the 14 week incubation period at 20/10 °C, whereas the length of the epicotyl–plumule increased only slightly during the first 8 weeks of incubation and then remained constant for the next 6 weeks (Fig. 6B). The length of cotyledons and of the epicotyl–plumule in radicle-emerged seeds increased only slightly during 16 weeks incubation at 5 °C. However, for radicle-emerged seeds incubated at 5 °C for 12 weeks and then at 20/10 °C for 2 weeks the length of both cotyledons and the epicotyl–plumule increased significantly, and cotyledons emerged from the seeds. Cotyledon length in seeds incubated for 8 or 12 weeks at 20/10 °C was significantly less (t-test, P < 0·001) than it was in seeds incubated at 5 °C, but the epicotyl–plumule length in seeds incubated for 4, 8 or 12 weeks at 20/10 °C was not statistically different from that of seeds incubated at 5 °C.
Observations on epicotyl–plumule development and cotyledon growth
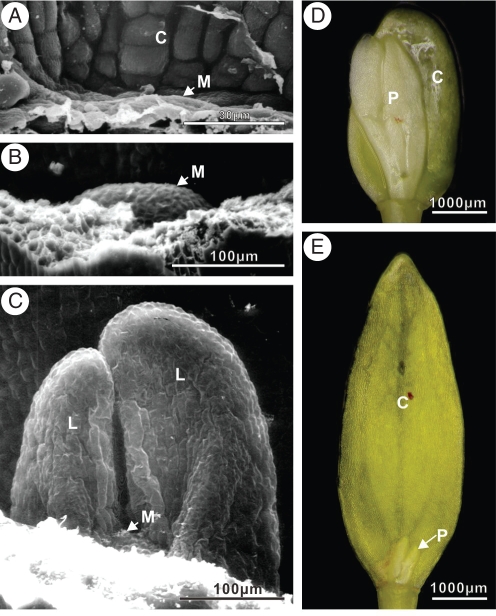
In freshly matured seeds of V. betulifolium, there was no evidence of the epicotyl or of activity of the apical meristem, i.e. no bulge of cells was present (Fig. 7A). A bulge had begun to form by the time the seed coat split, and the radicle had begun to emerge from the seed (Fig. 7B). The first true leaves had grown to about 200 µm by the time the radicle had emerged to a length of 1 mm (Fig. 7C), and the epicotyl could be observed. The epicotyl and plumule continued to grow slowly at 20/10 °C (Fig. 6A). Formation and growth of the apical meristem and first true leaves in V. parvifolium was similar to that observed in V. betulifolium.
Fig. 7.
Shoot apical meristem, epicotyl, plumule and cotyledon development in Viburnum betulifolium seeds collected in 2007. Scanning electron microscope (SEM) photograph of the shoot apical meristem in a fresh mature seed where the apical meristem will appear (arrow, A) and in an embryo at the time the seed coat splits at 20/10 °C, when the apical meristem began to grow (B). SEM photograph of developing first true leaves after radicle emergence to 1 mm in length (C). Dissecting microscope photograph of cotyledons and plumule (first true leaves) inside radicle-emerged seed incubated at 20/10 °C for 24 weeks (D) and at 5 °C for 20 weeks (E). Abbreviations: C, cotyledon; L, first true leaves; M, shoot apical meristem; P, plumule.
The length of the cotyledon and of the epicotyl–plumule remained nearly the same in radicle-emerged seeds incubated at 20/10 °C for 24 weeks (Fig. 7D). However, they were significantly longer inside radicle-emerged seeds incubated at 5 °C for 20 weeks (Fig. 7E), by which time the cotyledons had pushed through the endosperm and endocarp.
DISCUSSION
The optimum temperature for maximum germination (radicle emergence) percentage and rate of germination in seeds of V. betulifolium and of V. parvifolium was 20/10 °C, and seeds of both species began to germinate at this temperature regime after 16 and 18 weeks, respectively. Exposure of seeds to a sequence of temperatures from warm to cold or from cold to warm showed that the radicle always began to emerge at 20/10 °C. However, if seeds were initially exposed to cold in the temperature sequence, radicle emergence was delayed until the second exposure to 20/10 °C, after the seeds had been exposed to 25/15 °C. Furthermore, considerable embryo growth occurred at 25/15 °C and/or at 20/10 °C prior to radicle emergence. The small linear embryo in seeds of V. betulifolium and V. parvifolium increased in length by 650 and 640 %, respectively, before radicle emergence occurred. GA3 had no effect on radicle emergence. However, embryos, i.e. cotyledons and plumule, continued to grow inside the seeds after the radicle emerged. Shoot emergence from radicle-emerged seeds required exposure to 5 °C for ≥12 weeks (Fig. 6). Taken together, the seeds of V. betulifolium and V. parvifolium have deep simple epicotyl MPD (Baskin and Baskin, 2004). The formula for this kind of seed dormancy is C1bB (root)–C3 (shoot), where B indicates morphological dormancy, C1b non-deep PD (C1) that requires warm stratification (subscript b) for root emergence and C3 deep PD that requires a long period of cold stratification for shoot emergence (Baskin and Baskin, 2008).
Seeds of various temperate-zone species of Viburnum, including V. acerifolium (Giersbach, 1937; Hidayati et al., 2005), V. alnifolium, V. cassinoides, V. dentatum, V. dilatatum, V. lentago, V. opulus, V. prunifolium, V. rafinesquianum, V. rufidulum and V. trilobum (Giersbach, 1937), have deep simple epicotyl MPD. Seeds of all of these species required a long period of time at warm temperatures for root emergence and then a long period of time at cold temperatures for shoot emergence (Baskin et al., 2009a). However, seeds of V. tinus from Spain (Karlsson et al., 2005) and of V. odoratissimum (Baskin et al., 2008) have non-deep simple epicotyl MPD and require a relatively short period of warm stratification to break radicle dormancy and then another relatively short warm period to break shoot dormancy.
The epicotyl and apical meristem are not visible in the mature seeds of V. betulifolium or V. parvifolium. In addition to continued cotyledon growth in radicle-emerged seeds, the epicotyl grew and the apical meristem produced the first pair of true leaves inside the seed. Few studies on epicotyl dormancy have characterized shoot growth and in particular development of the first leaves in any detail, although growth of cotyledons in radicle-merged seeds has been documented (Baskin et al., 2009b). In seeds of Daphniphyllum glaucescens, which have non-deep simple epicotyl MPD, the cotyledons continue to grow inside radicle-emerged seeds for several weeks before the shoot emerges (Baskin et al., 2009b). Also, a small bud forms prior to shoot emergence in D. glaucescens, but the details of its development have not been studied. Seeds of Humboldtia laurifolia have a fully developed embryo but a kind of physiological epicotyl dormancy, in which the epicotyl and plumule develop inside the seed after the radicle emerges (Jayasuriya et al., 2010). Seeds of Chionanthus retusus also have a fully developed embryo and physiological epicotyl dormancy. In fresh seeds of C. retusus, the epicotyl was undetectable, but it began to grow after radicle emergence and emerged after about 3 months of incubation at a warm temperature (Chien et al., 2004). Thus, the radicles of C. retusus and H. laurifolia are non-dormant and emerge soon after seed dispersal, whereas the epicotyl and plumule continue to grow at warm temperature until the shoot emerges.
Once the radicle emerges, seeds of V. betulifolium and V. parvifolium require a long cold period at 5 °C before the shoot emerges. The reason the shoot did not emerge soon after the radicle is that initiation of development of the epicotyl and plumule is delayed until after seeds have been warm stratified. That is, the apical meristem in fresh seeds of V. betulifolium was flat; however, a bulge, indicating that the meristem had become active, was present at the time the embryo had grown enough to split the seed coat. After radicle emergence, epicotyl–plumule development continued at 5 °C and at 20/10 °C in both species, but the epicotyl–plumule emerged only from those radicle-emerged seeds that were cold stratified. In radicle-emerged seeds of P. suffructicosa, the epicotyl tissue also became differentiated both at 5 °C and in warm conditions (Barton and Chandler, 1957). However, as in the two Viburnum species the shoot emerged only in cold-treated radicle-emerged seeds.
In seeds with deep simple MPD, the breaking of PD is divided into two parts: one broken by warm and the other by cold stratification. Also, embryo growth occurs at high (or intermediate) temperatures after (or during) the time when the first part of PD is broken. In seeds of V. betulifolium and V. parvifolium, much of the embryo growth occurred at high temperature, after the seeds had been warm stratified for about 12 weeks. After a considerable amount of embryo growth and after radicle emergence, the second part of PD was broken by cold stratification in seeds of the two Viburnum species. In radicle-emerged seeds of V. betulifolium and V. parvifolium, continued cotyledon growth and formation of the plumule occurred at 20/10 and 5 °C, but only those radicle-emerged seeds cold treated at 5 °C lost the second part of PD and produced an emerged shoot. Epicotyl–plumules and cotyledons of radicle-emerged seeds grew more slowly in V. betulifolium than in V. parvifolium after they were kept at cold temperature (5 °C) for 12 weeks and then moved to warm temperature (20/10 °C) (Fig. 6). This indicates that the epicotyl–plumule in V. betulifolium may need more cold stratification for emergence, i.e. the second part of PD may be a little deeper in V. betulifolium than in V. parvifolium seeds.
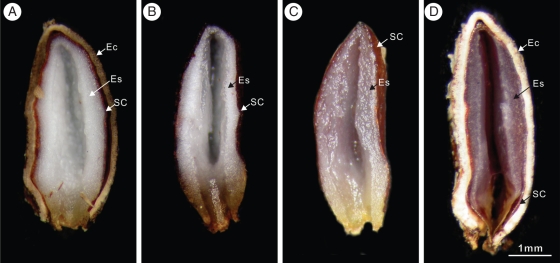
Seeds of V. opulus (Karimova et al., 2000), and presumably those of other members of the genus, contain high amounts of lipids and proteins. At cold, but not at warm, temperatures the endosperm in V. betulifolium and in V. parvifolium became soft and transparent, indicating that the storage material had been broken down (Fig. 8). Thus, rapid growth of the shoot of V. betulifolium and of V. parvifolium following cold stratification might be due to the activation of enzymes in the endosperm that degraded lipids and proteins, providing carbon and energy for shoot growth. However, essentially nothing is known about the biochemical mechanism(s) that control shoot emergence in Viburnum seeds or in seeds of other species with epicotyl MPD. After epicotyl dormancy was broken in seeds of P. suffruticosa during cold stratification at 5 °C, the amount of all amino acids and amides, except the amino acids aspartic, asparagine and arginine, had increased in the endosperm, and alanine, histidine and glutamine had increased in the embryo (Barton and Bray, 1967). In Heracleum sphondylium seeds, which have deep complex MPD, growth of the underdeveloped embryo and germination occurred at 5 °C. Low temperatures (2–5 °C) caused conversion of storage proteins into soluble nitrogenous compounds that apparently were used in growth of the embryo and in germination (Stokes, 1952, 1953a, b).
Fig. 8.
Dissecting microscope photographs of longitudinal sections of endosperm in radicle-emerged seeds of Viburnum betulifolium collected in 2007 and incubated in light at 20/10 °C for 12 (A) and 16 (B) weeks and at 5 °C in the dark for 12 (C) and 16 (D) weeks, when seeds were ready to germinate. Abbreviations: Ec, endocarp; Es, endosperm; SC, seed coat.
The ecological consequence of the presence of deep simple epicotyl MPD in seeds is that radicles and shoots do not emerge at the same time. Furthermore, a sequence of warm to cold to warm temperatures is required for emergence of both the radicle and shoot. Since emergence of the radicle occurs before that of the shoot and there is a delay of several weeks between radicle and shoot emergence, the root system would be established before the shoot emerged. A cold stratification requirement for shoot emergence means that the shoot cannot emerge until spring, but it is clear that shoots of cold-treated root-emerged seeds, e.g. those of V. betulifolium and V. parvifolium, can grow rapidly at spring temperatures. At the time of shoot emergence in spring, not only are the cotyledons of V. betulifolium and V. parvifolium fully elongated (inside the seed), but the first pair of leaves has been initiated. Thus, the shoot has both cotyledons and leaves that would be capable of photosynthetic activity as soon as they emerge from the seed.
Our hypothesis that the shoot would be well developed in spring is supported. On the other hand, the hypothesis that cold stratification is required for development of the shoot is not supported. Thus, additional cotyledon growth and formation of the plumule occurred at both 20/10 and 5 °C. However, even when development was completed, the second part of PD prevented shoot emergence. After the PD was broken by cold stratification, shoots emerged rapidly, especially at the elevated temperatures of spring.
ACKNOWLEDGEMENTS
The authors thank Wei-Lien Chen, Ta-Yuan Chien, Huei-Ping Huang, Alice Wu, Yen-Wei Chang, Chang-Yen Chen, Wen-Yu Hsu and Kai-Chun Yang for technical assistance. This research was supported by grants NSC 99-2628-B-054-001-MY3 to C.-T.C., NSC 89-2311-B-002–063 to L.-L.K.-H. and 99AS-8·1·2-F1-G1 to C.-T.C.. We thank the National Science Council (NSC) and the Council of Agriculture, Executive Yuan, Taiwan, for this support.
LITERATURE CITED
- Barton LV, Bray JL. Biochemical studies of dormancy and after-ripening of seeds. IV. Further studies on changes in contents of some amino acids and organic acids. Contributions from Boyce Thompson Institute. 1967;23:311–318. [Google Scholar]
- Barton LV, Chandler C. Physiological and morphological effects of gibberellic acid on epicotyl dormancy in tree peony. Contributions from Boyce Thompson Institute. 1957;19:201–214. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin CC, Baskin JM. When breaking seed dormancy is a problem, try a move-along experiment. Native Plants Journal. 2003;4:17–21. [Google Scholar]
- Baskin CC, Chien CT, Chen SY, Baskin JM. Germination of Viburnum odoratissimum seeds: a new level of morphophysiological dormancy. Seed Science Research. 2008;18:179–184. [Google Scholar]
- Baskin CC, Chen SY, Chien CT, Baskin JM. Overview of seed dormancy in Viburnum (Caprifoliaceae) Propagation of Ornamental Plants. 2009a;9:115–121. [Google Scholar]
- Baskin CC, Chien CT, Chen SY, Baskin JM. Epicotyl morphophysiological dormancy in seeds of Daphniphyllum glaucescens, a woody member of the Saxifragales. International Journal of Plant Sciences. 2009b;170:174–181. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC. Some considerations for adoption of Nikolaeva's formula system into seed dormancy classification. Seed Science Research. 2008;18:131–137. [Google Scholar]
- Baskin JM, Hidayati SN, Baskin CC, Walck JL, Huang ZY, Chien CT. Evolutionary considerations of the presence of both morphophysiological and physiological seed dormancy in the highly advanced euasterids II order Dipsacales. Seed Science Research. 2006;16:233–242. [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. 2nd edn. New York: Plenum Press; 1994. [Google Scholar]
- Chien CT, Kuo-Huang LL, Shen YC, Zhang R, Chen SY, Yang JC, Pharis RP. Storage behavior of Chionanthus retusus seed and asynchronous development of the radicle and shoot apex during germination in relation to germination inhibitors, including abscisic acid and four phenolic glucosides. Plant and Cell Physiology. 2004;45:1158–1167. doi: 10.1093/pcp/pch129. [DOI] [PubMed] [Google Scholar]
- Giersbach J. Germination and seedling production of species of Viburnum. Contributions from Boyce Thompson Institute. 1937;9:79–90. [Google Scholar]
- Hidayati SN, Baskin JM, Baskin CC. Epicotyl dormancy in Viburnum acerifolium (Caprifoliaceae) American Midland Naturalist. 2005;153:232–244. [Google Scholar]
- International Seed Testing Association. International rules for seed testing. Seed Science and Technology. 1999;27 (Suppl.):1–333. [Google Scholar]
- Jayasuriya KMGG, Wijetunga ASTB, Baskin JM, Baskin CC. Recalcitrancy and a new kind of epicotyl dormancy in seeds of the understory tropical rainforest tree Humboldtia laurifolia (Fabaceae, Ceasalpinioideae) American Journal of Botany. 2010;97:15–26. doi: 10.3732/ajb.0900213. [DOI] [PubMed] [Google Scholar]
- Karimova AR, Yunusova SG, Maslennikov SI, Galkin EG, Yunusov TS, Shereshovets VV, Yunusov MS. Lipids, lipophilic components, and biologically active fractions of Viburnum opulus L. seeds. Chemistry of Natural Compounds. 2000;36:560–564. [Google Scholar]
- Karlsson LM, Hidayati SN, Walck JL, Milberg P. Complex combination of seed dormancy and seedling development determine emergence of Viburnum tinus (Caprifoliaceae) Annals of Botany. 2005;95:323–330. doi: 10.1093/aob/mci029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Okubo N, Miura T, Honda K, Ishikawa Y. Ecophysiology of seed germination in Erythronium japonicum (Liliaceae) with underdeveloped embryos. American Journal of Botany. 2002;89:1779–1784. doi: 10.3732/ajb.89.11.1779. [DOI] [PubMed] [Google Scholar]
- Kondo T, Miura T, Okubo N, Shimada M, Baskin CC, Baskin JM. Ecophysiology of deep simple epicotyl morphophysiological dormancy in seeds of Gagea lutea (Liliaceae) Seed Science Research. 2004;14:371–378. [Google Scholar]
- Nikolaeva MG. Physiology of deep dormancy in seeds. 1969 Leningrad: Izdatel'stvo ‘Nauka’. (Translated from Russian by Z. Shapiro, National Science Foundation, Washington, DC.) [Google Scholar]
- Stokes P. A physiological study of embryo development in Heracleum sphondylium L. I. The effect of temperature on embryo development. Annals of Botany. 1952;16:441–447. [Google Scholar]
- Stokes P. A physiological study of embryo development in Heracleum sphondylium L. III. The effect of temperature on metabolism. Annals of Botany. 1953a;17:157–173. [Google Scholar]
- Stokes P. The stimulation of growth by low temperature in embryos of Heracleum sphondylium L. Journal of Experimental Botany. 1953b;4:222–234. [Google Scholar]
- Wang BSP, Lin TP, Chang TT. Control of fungal growth with sphagnum for cold stratification and germination of tree seeds. Taiwan Journal of Forest Research. 1998;13:101–108. [Google Scholar]
- Yang KC, Chiu ST. Flora of Taiwan. 2nd edn. Vol. 4. Taipei, Taiwan: Editorial Committee of the Flora of Taiwan; 1998. Caprifoliaceae. In: Editorial Committee of the Flora of Taiwan; pp. 738–759. [Google Scholar]