Abstract
Biogenic amine systems are damaged by amphetamine abuse and in Parkinson's disease. The mechanisms mediating this damage are of high importance because of the public health impact of these problems. Here we have taken advantage of the C. elegans nematode model system to investigate genetic modifiers of biogenic amine toxicity. In a forward genetic screen, we identified a mutant resistant to the toxic effects of dopamine. This mutant was also resistant to toxic doses of methamphetamine (MA) and 3,4-methylenedioxymethamphetamine (MDMA). In addition, this mutation conferred resistance to 6-hydroxydopamine damage to dopaminergic neurons in a Parkinson's disease model. Resistance was due to a mutation in the nsy-1 gene, orthologous to the mammalian ASK-1 MAPKKK. NSY-1 is in the highly conserved p38 MAP kinase pathway, which plays a crucial role in C. elegans innate immunity, suggesting that this pathway may play a role in biogenic amine toxicity system damage due to amphetamines and in the pathogenesis of Parkinson's disease in higher organisms.
Keywords: Caenorhabditis elegans, methamphetamine, MDMA, p38, innate immunity
Introduction
Damage to biogenic amine neural systems occurs in both Parkinson's disease (PD) and with abuse of amphetamines, particularly methamphetamine (MA) and 3,4-methylenedioxymethamphetamine (MDMA) [2, 27, 30]. To explore the molecular mechanisms of this damage, we have used C. elegans nematodes. C. elegans is a versatile model system which has been used to model PD with 6-hydoxydopamine (6-OHDA)-induced dopaminergic neurodegeneration [4, 16, 21, 35], which is dependent on the dopamine transporter as it is in mammals [20]. C. elegans has also been used productively to study drugs which affect biogenic amine systems. Mutants resistant to the effects of fluoxetine have been identified, helping to clarify its complex mechanisms of action [7, 11, 15, 23]. Cocaine and amphetamine, stimulants which affect biogenic amine systems, also produce effects on C. elegans, which are mediated by conserved dopamine receptors and the dopamine transporter [5, 36].
Taken together, this work indicates that C. elegans is useful for identifying genetic modulators involved in amphetamine effects and in PD models. Here, we examined the behavioral effects of the two most highly abused drugs in the amphetamine class, methamphetamine (MA) and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). To our knowledge, this is the first demonstration of these abused drugs' effects on this widely used model organism. We found that prolonged exposure to these drugs is toxic, and in a screen for mutants resistant to the lethal effects of exogenous dopamine, we identified a novel genetic modulator providing resistance to toxicity from dopamine, amphetamines, and 6-OHDA.
Material and Methods
Strains used were wild-type Bristol N2, and mutant strains BZ981 nsy-1(eg691); AU3 nsy-1(ag3); KU4 sek-1(km4); KU25 pmk-1(km25); and BS3383 pmk-3(ok169). A transgenic rescue construct was made from a 3.3 kb nsy-1 promoter fused to the nsy-1 genomic sequence encoding the mutant eg691 allele. For visualizing dopamine neurons, Pdat-1∷GFP (strain BY200, courtesy of Randy Blakely [21]) was used; for visualizing serotonin neurons, Ptph-1∷GFP (stain SK4013, courtesy of Scott Clark [8]) was used.
MA and MDMA experiments used methamphetamine (Sigma) or MDMA (Drug Supply Program, National Institute on Drug Abuse). 1M stock solution was added to melted noble agar at approximately 55°C and poured into plates. For lethality assays, plates were seeded with concentrated E. coli OP50 suspensions in M9 buffer (20 μl) and dried overnight in the dark. The following day, a palmitate barrier (30 μl of 10 mg/ml solution in ethanol) was added at the edge of the plate to prevent animals from leaving the drug exposure agar, and allowed to dry. First day adults were placed on plates in groups, then plates were sealed and stored at room temperature (22°C) in the dark overnight and scored for survival at 16 hours of exposure.
For behavioral tests, all animals tested were the same age (first day adults). For all behavior assays, a suspension of E. coli (50 μl) was placed in the center of drug plates and allowed to air-dry. For methamphetamine behavioral assays, the palmitate barrier described above was added. In each independent data point, ten animals were examined after one hour of exposure to drug. For locomotion, each animal in a group of ten was scored for spontaneous movement in a ten second window. The number moving in each group was converted to a percentage for each independent test of groups of ten animals. For egg-laying, ten animals were placed on the plate and allowed to lay eggs, then removed and eggs were counted. For feeding, ten animals were placed on plates with food and observed for pharyngeal pumping behavior indicative of active feeding; the number pumping out of ten animals was converted to a percentage for each independent group of ten. Significance is indicated for P < .05 with Bonferroni correction (except for 6-OHDA results which used an uncorrected two-tailed t-test); N for all experiments is given in Supplementary Data 1.
In a screen for resistance to lethality from dopamine exposure, animals were mutagenized using standard methods with ethylmethanesulfonate (EMS, Sigma) at a concentration of 50 mM. Animals in the second generation (i.e., F2 corresponding to approximately 2500 genomes total) were screened on concentrations of dopamine that produce 100% lethality in wild-type to identify potential resistant mutants. A candidate strain, BZ981 (eg691), was isolated and outcrossed six times to wild-type N2, with no change in phenotype. Standard single-nucleotide polymorphism mapping was used with Hawaiian stain CB4856, taking advantage of Dra1 restriction sites [10]. After mapping to a small genetic interval, nsy-1 was selected as a candidate gene. Sequencing of nsy-1(eg691) revealed a single nucleotide mutation from G to A, resulting in a predicted codon change from ATG (M) to ATA (I) at position 1217 of the C. elegans NSY-1 protein.
To produce specific dopaminergic lesions, animals were exposed to 6-hydroxydopamine (Sigma) as previously described [21]. Animals were scored as either having intact dopaminergic neural processes, or, if any damage or discontinuity of these processes was observed, as not having intact dopamine neurons.
Results and Discussion
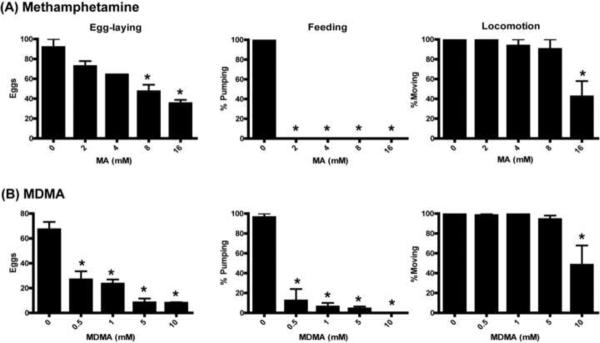
To lay a foundation for the use of C. elegans in studying highly abused amphetamines, we examined the behavioral effects of MA and MDMA (Fig. 1). Acute exposure (one hour) led to pronounced changes in behavior with both drugs; shorter exposures produced graded effects, with differences in timing suggesting that these effects may depend on receptors with different affinities or kinetics for the drugs (Supplementary Figure 1). MA produced dramatic reductions in crawling leading to paralysis, reduction of feeding as indicated by decreased pumping of the pharyngeal bulb, and suppressed egg-laying (Fig. 1A). This pattern is very similar to the behavioral effects of exogenous dopamine [6, 17, 25, 26], consistent with findings that the effects of amphetamine, the parent compound in this class of drugs, are mediated by dopamine in C. elegans [5]. MDMA produced subtly different effects, also inhibiting feeding and producing paralysis, but having less effect on egg-laying (Fig. 1B). It is possible that this is due to complex interaction with multiple biogenic amine systems in C. elegans, as MDMA is known to act through dopamine, serotonin, and norepinephrine systems in mammals [29, 37]. Differences in the sensitivity to amphetamines on locomotion versus egg-laying and feeding may be due to underlying differences in the sensitivity of the receptors responsible for these effects, or to differences in the balance of receptor types, for example octopamine, dopamine, and serotonin receptors, being affected by the drugs.
Figure 1.
Acute behavioral effects of MA and MDMA. (A) Effects of methamphetamine on egg-laying, feeding (pharyngeal pumping), and locomotion. (B) Effects of MDMA on egg-laying, feeding, and locomotion. First day adult animals were examined after one hour of drug exposure at the concentrations indicated. For all Figures, data shown are mean and SEM; (*) indicates P<.05 with multiple comparison Bonferroni correction.
We also observed MDMA to produce a novel behavioral effect on locomotion in which affected animals turned continuously, in contrast to the typical onset of paralysis in which only slowing of locomotion is observed. In this behavior, the animals' head was held locked in a straight position, and the “neck” region, immediately behind the head, was bent, resulting in circling. This behavior was predominantly, if not exclusively, toward the ventral direction. This suggests an unusual asymmetry of action which may be of interest for future investigation, as there are relatively few asymmetric aspects of the C. elegans body and nervous system. These results indicate that C. elegans behavior responds strongly to these abused substances. Genetic screens could be utilized to isolate mutants resistant to these effects, identifying new genes involved in these drugs' mechanism of action as has been proposed previously for cocaine [36] and amphetamine [5]. Rather than these acute behavioral effects, here we chose to focus on the effects of longer exposures, to determine whether this system can be used to study these substances' toxicity.
Because MDMA preferentially accumulates in 5-HT neurons and leads to neuronal damage [2, 27, 30], we examined the integrity of 5-HT neurons after exposure to MDMA in varying concentrations (up to 30 mM) and durations (up to 3 hours). Despite testing this wide range of conditions, we did not detect any specific damage to 5-HT neurons suggestive of degeneration as seen in mammalian systems. This may be due to true species differences, or inherent limitations of our assay, such as a lack of sensitivity of the microscopic examination.
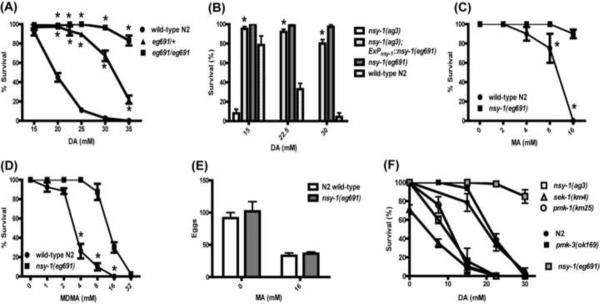
Longer exposures (16 hours) to MA and MDMA resulted in dose-dependent toxicity and lethality in these animals (Fig. 2). We also noted this effect with prolonged exposure to exogenous dopamine (DA) at similar concentrations to those used previously for behavioral studies [6]. In a screen using dopamine-induced lethality, we isolated a mutant, eg691, showing strong resistance to the lethal effects of exogenous dopamine (Fig. 2A). Note that in this screen, dopamine was used, and mutants were subsequently tested for lethality due to MA and MDMA; using dopamine, novel targets that specifically play a role in MA and MDMA toxicity, but not DA toxicity, may be missed, and it would be of interest in future experiments to perform screens using these drugs' lethality independent of dopamine.
Figure 2.
Lethal effects of prolonged exposure to MA, MDMA, and dopamine (DA). (A–B) (A) Survival after prolonged (16 hour) exposure to dopamine. The nsy-1(eg691) allele is semi-dominant for resistance to dopamine-induced lethality. (B) Expression of the nsy-1(eg691) gene restores resistance in the nsy-1(ag3) loss-of-function background, which is hypersensitive to dopamine-induced lethality. (C–D) Survival with long exposure to methamphetamine (C) or MDMA (D). (E) Egg-laying behavior of wild-type N2 versus nsy-1(eg691) on MA. The mutant shows similar drug-induced inhibition of egg-laying as wild-type. (F) Genetic analysis of dopamine-induced lethality. Predicted reduction of function alleles in the PMK-1 p38 MAPK pathway show enhanced sensitivity compared to wild-type or nsy-1(eg691) presumed gain-of-function allele. For tests of statistical significance, see Supplementary Data 1.
Using standard genetic mapping techniques [10], we identified a single base change in the nsy-1gene in the mutant eg691 allele. This change is predicted to result in a coding change from methionine to isoleucine at amino acid position 1217. This mutation lies near the C-terminal of the protein, far from the conserved kinase domain. Although this region shows a high degree of sequence conservation with the mammalian orthologue of NSY-1, it is not related to any previously characterized structural domains. Previously, the nsy-1 gene was identified in C. elegans for its effect on neuronal identity specification in development; the gene name nsy-1 refers to non-symmetrical neuronal fates in a specific sensory neuron [24, 31, 34]. However, nsy-1 also has functions in innate immunity [14], and is highly conserved with its mammalian orthologue, the MAPKKK ASK-1 (apoptosis signal-regulating kinase-1).
Genetic analysis showed that the nsy-1(eg691) mutant resistance phenotype was semi-dominant. Heterozygotes exhibited an intermediate susceptibility to exogenous dopamine-induced lethality compared to the phenotype of homozygous mutants (Fig. 2A). This is suggestive of a gain of function phenotype, so we tested an already-available loss of function allele, nsy-1(ag3) [14]. This reduction of function allele showed a phenotype opposite that of nsy-1(eg691), with a strongly enhanced sensitivity to dopamine toxicity (Fig. 2B and 3A). This enhancement is similar to the increased susceptibility to pathogenic bacterial infection reported in the nsy-1 loss of function [14]; as with enhanced sensitivity to killing by a bacterial pathogen, this mutant showed increased sensitivity to exogenous dopamine. This suggests that, as indicated by genetic analysis, levels of function in NSY-1 correlate well with the toxicity of exogenous dopamine. Consistent with this, transgenic expression of the nsy-1(eg691) mutant gene in the reduction of function background nsy-1(ag3) restored full resistance to dopamine toxicity (Fig. 2B). Speculatively, the eg691 mutation may result in a gain of function due to loss of repression of NSY-1 function, as MAPK are tightly regulated proteins. Further biochemical examination of the basis for this gain of function would be of interest, for example to determine if the mutation interferes with regulation of NSY-1 by other kinases.
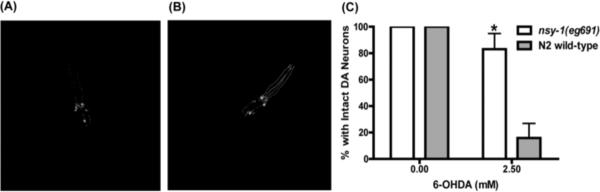
Figure 3.
Effects of 6-OHDA on dopamine neurons in nsy-1(eg691) versus wild-type N2. (A–B) Examples of effects of 6-OHDA on dopamine neuron processes in the C. elegans head (CEP neurons). (A) Damage to dopaminergic neuron processes in a wild-type animal after exposure to 6-OHDA. (B) Intact dopaminergic neurons in a nsy-1(eg691) animal after the same exposure to 6-OHDA as in (A). Processes are unaffected in this individual. (C) Comparison of effects of 6-OHDA on dopamine neurons in wild-type N2 and nsy-1(eg691) mutants, which are significantly resistant (p<.05).
Studies with wild-type nematodes indicated that dopamine lethality was a good indicator of sensitivity to lethality from MA and MDMA as well. Thus, we tested nsy-1(eg691) animals for lethality with MA and MDMA, and as with dopamine, the mutant was significantly resistant to the lethal effects of prolonged exposure to both of these amphetamines (Fig. 2C, 2D). This suggests a possible common mechanism of this toxicity among all of these biogenic amine-related substances. To test whether resistance to the lethal effects of biogenic amines corresponded with changes in behavioral susceptibility, we also assessed whether nsy-1(eg691) had an effect on the egg-laying response to methamphetamine (Fig. 2E). In contrast to the dramatic difference between wild-type and mutant with regard to the lethal effect of prolonged exposure, the behavioral egg-laying response was similarly inhibited in both strains.
Next, we examined additional mutants in the NSY-1 pathway (Fig. 2F). Reduction of function mutants for the MAPKK orthologue sek-1 [24, 31], and the p38 MAP kinase orthologue pmk-1 [1, 3] showed enhanced sensitivity to dopamine toxicity, similar to the nsy-1(ag3) mutant. This parallels the effects of these mutants on innate immunity. In contrast, a mutant of pmk-3, a distinct p38 isoform in C. elegans thought to act via a different pathway and to subserve functions distinct from innate immunity [3, 12, 19], did not show this enhanced sensitivity (Fig. 2F). This suggests a degree of specificity for the PMK-1 p38 MAP kinase pathway in determining sensitivity to exogenous biogenic amine toxicity.
To test whether resistance to lethality extended specifically to dopamine neurons in a common PD model, we compared 6-OHDA neurotoxicity in wild-type versus nsy-1(eg691) mutants and found significant resistance in these mutants to damage to dopamine neuron processes (Fig. 4). This suggests that even in the context of a more specific toxin for dopamine neurons, the nsy-1(eg691) allele provides resistance.
We have characterized the behavioral effects of methamphetamine and MDMA on C. elegans, suggesting this genetically tractable system will be useful for studying these abused drugs' mechanisms, complementing previous findings with amphetamine and cocaine [5, 36]. We also used the lethality of prolonged exposure to dopamine to identify a mutation in NSY-1, an upstream kinase in the conserved p38 MAP kinase pathway, which produced substantial resistance. This is likely due to a gain of function, as the phenotype is opposite to that of the NSY-1 loss of function. These results are surprising, as the mammalian NSY-1 orthologue ASK-1 responds to cellular threats such as oxidative stress and free radicals by triggering a biochemical cascade leading to p38 activation and ultimately apoptosis [32]. More specific to biogenic amine pathophysiology, the p38 pathway is regulated by DJ-1, which is disrupted in some familial PD [18]; in this work, decreased DJ-1 function upregulated ASK-1 kinase and led to p38 pathway activation and dopaminergic neuron death. These mechanisms may be conserved, as C. elegans DJ-1 has also been connected to the p38 pathway [9]. In another PD model, 6-OHDA activated ASK-1 and led to apoptosis in mammalian cells in vitro [22]. Finally, over-expression of tyrosinase, which produces excess dopamine-related oxidative toxicity, also activates p38 and results in cellular death [13]. These results implicate a toxic effect of p38 MAP kinase pathway activation. In contrast, in the present results, this pathway provided enhanced resistance to toxicity. To account for this, we suggest a model based on the function of this pathway in innate immunity, where it protects from pathogenic bacteria, and loss of function mutations in this pathway enhance susceptibility to pathogens [1, 14, 28, 33]. Analogously, we propose that basal increased activity of NSY-1, perhaps at more modest levels than under intense oxidative stress, protects from biogenic amine lethality by providing enhanced innate immune-like function.
Interesting questions for future work include examining this pathway's activity biochemically and the mechanisms of MA and MDMA toxicity, especially whether oxidative stress, proteostasis (perhaps itself due to oxidative stress), or other mechanisms lead to death. In particular, it will be important to determine which tissues play a role in NSY-1-mediated resistance to biogenic amine toxicity. NSY-1 promoter-GFP fusions drove expression in intestine, hypodermis, and multiple as-yet unidentified neurons [24], which suggests that these tissues may be critical for this effect. An especially interesting question is whether the effects of 6-OHDA are cell-autonomous.
Our results suggest that manipulating the activity of the p38 MAP kinase pathway may be one way to produce resistance to biogenic amine-related insults, both from amphetamines and in a model of PD. Because the constituents of this pathway are highly conserved, it will be of interest to manipulate them in mammalian models to test whether they might show promise in combating these common and devastating clinical problems.
Supplementary Material
Research highlights
We examine the effects of methamphetamine and MDMA on C. elegans.
A p38 MAP kinase pathway mutant provides resistance to methamphetamine and MDMA toxicity.
Innate immunity may protect against pathologic processes involving biogenic amines.
Acknowledgments
Thanks to the NIDA Drug Supply Program for supplying MDMA. Strains were obtained from the Caenorhabditis Genetics Center, funded by the NIH National Center for Research Resources (NCRR). This research was supported by the Molecular Approaches to Mental Illness Fellowship in the UCSF Department of Psychiatry, and through funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Information.
Supplementary Data 1. Data on experimental numbers of animals and independent data points for each result shown in Figures.
Supplementary Figure 1. Time course of behavioral action of methamphetamine and MDMA.
References
- [1].Aballay A, Drenkard E, Hilbun LR, Ausubel FM. Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol. 2003;13:47–52. doi: 10.1016/s0960-9822(02)01396-9. [DOI] [PubMed] [Google Scholar]
- [2].Battaglia G, Yeh SY, O'Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- [3].Berman K, McKay J, Avery L, Cobb M. Isolation and characterization of pmk-(1–3): three p38 homologs in Caenorhabditis elegans. Mol Cell Biol Res Commun. 2001;4:337–344. doi: 10.1006/mcbr.2001.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caldwell GA, Caldwell KA. Traversing a wormhole to combat Parkinson's disease. Dis Model Mech. 2008;1:32–36. doi: 10.1242/dmm.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carvelli L, Matthies DS, Galli A. Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol Pharmacol. 2010;78:151–156. doi: 10.1124/mol.109.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- [7].Choy RK, Thomas JH. Fluoxetine-resistant mutants in C. elegans define a novel family of transmembrane proteins. Mol Cell. 1999;4:143–152. doi: 10.1016/s1097-2765(00)80362-7. [DOI] [PubMed] [Google Scholar]
- [8].Clark SG, Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development. 2003;130:3781–3794. doi: 10.1242/dev.00571. [DOI] [PubMed] [Google Scholar]
- [9].Cornejo Castro EM, Waak J, Weber SS, Fiesel FC, Oberhettinger P, Schutz M, Autenrieth IB, Springer W, Kahle PJ. Parkinson's disease-associated DJ-1 modulates innate immunity signaling in Caenorhabditis elegans. J Neural Transm. 2010;117:599–604. doi: 10.1007/s00702-010-0397-4. [DOI] [PubMed] [Google Scholar]
- [10].Davis MW, Hammarlund M, Harrach T, Hullett P, Olsen S, Jorgensen EM. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics. 2005;6:118. doi: 10.1186/1471-2164-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dempsey CM, Mackenzie SM, Gargus A, Blanco G, Sze JY. Serotonin (5HT), fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg-laying behavior. Genetics. 2005;169:1425–1436. doi: 10.1534/genetics.104.032540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hasegawa T, Treis A, Patenge N, Fiesel FC, Springer W, Kahle PJ. Parkin protects against tyrosinase-mediated dopamine neurotoxicity by suppressing stress-activated protein kinase pathways. J Neurochem. 2008;105:1700–1715. doi: 10.1111/j.1471-4159.2008.05277.x. [DOI] [PubMed] [Google Scholar]
- [14].Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, Ausubel FM. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- [15].Kullyev A, Dempsey CM, Miller S, Kuan CJ, Hapiak VM, Komuniecki RW, Griffin CT, Sze JY. A genetic survey of fluoxetine action on synaptic transmission in Caenorhabditis elegans. Genetics. 2010;186:929–941. doi: 10.1534/genetics.110.118877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem. 2003;86:165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- [17].McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mo JS, Jung J, Yoon JH, Hong JA, Kim MY, Ann EJ, Seo MS, Choi YH, Park HS. DJ-1 modulates the p38 mitogen-activated protein kinase pathway through physical interaction with apoptosis signal-regulating kinase 1. J Cell Biochem. 2010;110:229–237. doi: 10.1002/jcb.22530. [DOI] [PubMed] [Google Scholar]
- [19].Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- [20].Nass R, Hahn MK, Jessen T, McDonald PW, Carvelli L, Blakely RD. A genetic screen in Caenorhabditis elegans for dopamine neuron insensitivity to 6-hydroxydopamine identifies dopamine transporter mutants impacting transporter biosynthesis and trafficking. J Neurochem. 2005;94:774–785. doi: 10.1111/j.1471-4159.2005.03205.x. [DOI] [PubMed] [Google Scholar]
- [21].Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ouyang M, Shen X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2006;97:234–244. doi: 10.1111/j.1471-4159.2006.03730.x. [DOI] [PubMed] [Google Scholar]
- [23].Ranganathan R, Sawin ER, Trent C, Horvitz HR. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci. 2001;21:5871–5884. doi: 10.1523/JNEUROSCI.21-16-05871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sagasti A, Hisamoto N, Hyodo J, Tanaka-Hino M, Matsumoto K, Bargmann CI. The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell. 2001;105:221–232. doi: 10.1016/s0092-8674(01)00313-0. [DOI] [PubMed] [Google Scholar]
- [25].Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- [26].Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- [27].Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1987;240:1–7. [PubMed] [Google Scholar]
- [28].Shapira M, Tan MW. Genetic analysis of Caenorhabditis elegans innate immunity. Methods Mol Biol. 2008;415:429–442. doi: 10.1007/978-1-59745-570-1_25. [DOI] [PubMed] [Google Scholar]
- [29].Steele TD, McCann UD, Ricaurte GA. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”): pharmacology and toxicology in animals and humans. Addiction. 1994;89:539–551. doi: 10.1111/j.1360-0443.1994.tb03330.x. [DOI] [PubMed] [Google Scholar]
- [30].Stone DM, Hanson GR, Gibb JW. Differences in the central serotonergic effects of methylenedioxymethamphetamine (MDMA) in mice and rats. Neuropharmacology. 1987;26:1657–1661. doi: 10.1016/0028-3908(87)90017-7. [DOI] [PubMed] [Google Scholar]
- [31].Tanaka-Hino M, Sagasti A, Hisamoto N, Kawasaki M, Nakano S, Ninomiya-Tsuji J, Bargmann CI, Matsumoto K. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 2002;3:56–62. doi: 10.1093/embo-reports/kvf001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Troemel ER, Sagasti A, Bargmann CI. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell. 1999;99:387–398. doi: 10.1016/s0092-8674(00)81525-1. [DOI] [PubMed] [Google Scholar]
- [35].van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ward A, Walker VJ, Feng Z, Xu XZ. Cocaine modulates locomotion behavior in C. elegans. PLoS ONE. 2009;4:e5946. doi: 10.1371/journal.pone.0005946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.