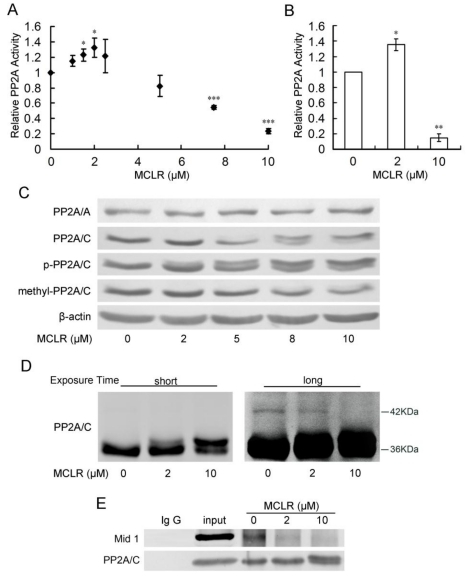
Figure 2.
PP2A shows opposing activity with diffuse alternations of its core enzyme protein level and post-translational modification under MCLR treatment. HEK293 cells were exposed to varying concentrations of MCLR for 24 h before PP2A analysis. (A) PP2A activity was measured with a Promega serine/threonine phosphatase kit assay. Data represent means ±SD (n=3) *, p<0.05; **, p<0.01; ***, p<0.001 compared with control. (B) PP2A activity was measured by PP2A immunoprecipitation phosphatase assay. Data represent means ±SD (n=3) *, p<0.05; **, p<0.01 compared with control. (C) PP2A core enzyme subunits A and C, including phosphorylated and methylated subunit C, were examined by Western blotting, where β-actin was used as loading control. (D) Ubiquitination of PP2A/C was captured by Western blotting with an antibody against PP2A/C at different exposure time (short: 1min, long: 10min). (E) Immunoprecipitation assay was applied in HEK293 cell lysate using an anti-PP2A/C antibody. The association of PP2A/C with Mid 1 form precipitated material was detected by Western blotting.