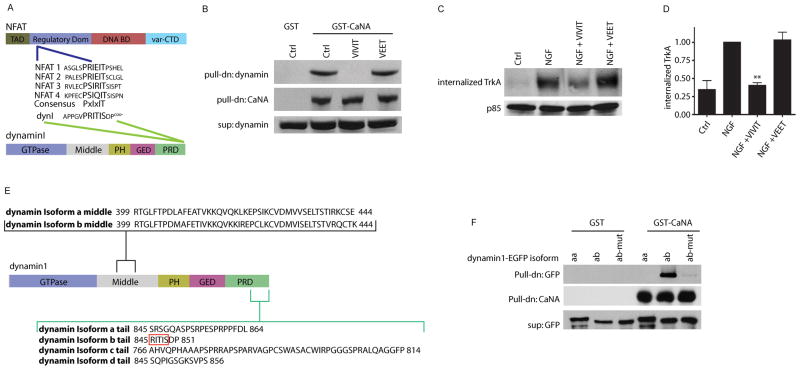
Figure 6. Calcineurin-dynamin1 interaction is mediated by a PxIxIT motif found in specific dynamin1 isoforms.
(A) Schematic of PxIxIT box consensus sequence found in the regulatory domain of NFAT (1–4) transcription factors and the PRITIS sequence in the proline-rich domain (PRD) of dynamin1. TAD is the transactivation domain, DNA BD is the DNA binding domain, var-CTD is the variable C-terminal domain for NFAT. PH is the pleckstrin homology domain and GED is the GTPase effector domain for dynamin. (B) Calcineurin-dynamin1 interaction is dependent on the PxIxIT motif. VIVIT peptide (a PxIxIT box mimic), but not a control VEET peptide, blocks association of CaNA with dynamin1. Pulldown with GST alone is shown as control. (C) Calcineurin-dynamin1 interaction via the PxIxIT motif is required for NGF-dependent TrkA internalization. Cell surface biotinylation assay shows that VIVIT, but not VEET treatment decreases NGF-dependent internalization of TrkA receptors. Supernatants were probed for p85. (D) Densitometric quantification of internalized TrkA, **p<0.01, n=4 (E, F) Calcineurin interaction is specific to dynamin1 variants with a PxIxIT box. (E) Schematic of dynamin1 splicing variants. Red box indicates xIxIS portion of the PRITIS box sequence, only present in b tail isoforms. (F) GST pull-down assays with HEK293 lysates show that calcineurin interacts with dynamin1ab via the PxIxIT box, but not dynamin1aa isoforms. HEK293 cells were transfected with dynamin1aa-EGFP, dynamin1ab-EGFP, or dynamin1ab-EGFP with PRITIS sequence mutated to ARATAA.