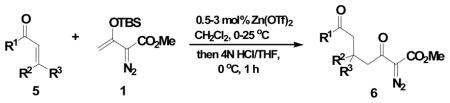
Table 2.
Zinc Triflate-Catalyzed Mukaiyama-Michael Reactions of 1 with Representative Enonesa
 | ||||
|---|---|---|---|---|
| entry | enone 5 | prduct 6 | yieldb, % | |
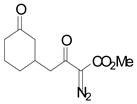
| 1 | a |
|
|
96 |
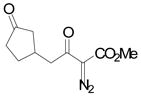
| 2 | b |
|
|
84 |
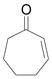
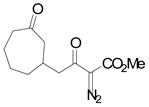
| 3 | c |
|
|
94 |
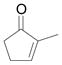
| 4c | d |
|
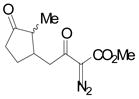
|
99 |
| 5d | e |
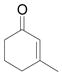
|
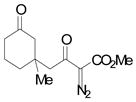
|
68 |
| 6e | f |
|
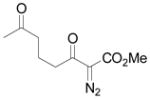
|
74 |
| 7d,f | g |
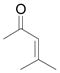
|
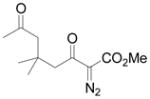
|
71 |
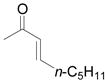
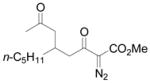
| 8 | h |
|
|
88 |
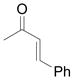
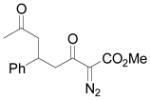
| 9 | i |
|
|
92 |
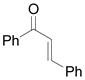
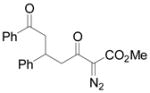
| 10 | j |
|
|
99 |
Vinyldiazoacetate 1 (0.75 mmol) was added to a solution of enone 5 (0.5 mmol) and zinc triflate (0.015 mmol) in CH2Cl2 (2 mL). The solution was stirred at 0°C and then allowed to warm to room temperature. After 16 hours, the crude reaction mixture was concentrated and then hydrolyzed with 1:5 4N HCl/THF at 0°C. See Supporting Information for details
Yield of isolated 6 following column chromatography.
The product was isolated as two diastereomers in 3:1 ratio.
Reaction time was 36 hours.
Two equivalents of 1 was used, 62% yield with 1.5 equiv. of 1; 71% yield with 0.5 mol % of catalyst.
Reaction was performed in 1 mL CH2Cl2, and the concentration of enone 5g was 0.5 M; 78% yield with 0.5 mol % catalyst.
