Figure 1.
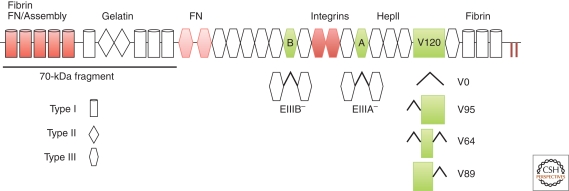
FN domain organization and isoforms. Each FN monomer has a modular structure consisting of 12 type I repeats (cylinders), 2 type II repeats (diamonds), and 15 constitutive type III repeats (hexagons). Two additional type III repeats (EIIIA and EIIIB, green) are included or omitted by alternative splicing. The third region of alternative splicing, the V region (green box), is included (V120), excluded (V0), or partially included (V95, V64, V89). Sets of modules comprise domains for binding to other extracellular molecules as indicated. Domains required for fibrillogenesis are in red: the assembly domain (repeats I1-5) binds FN, III9-10 contains the RGD and synergy sequences for integrin binding, and the carboxy-terminal cysteines form the disulfide-bonded FN dimer (‖). The III1-2 domain (light red) has two FN binding sites that are important for fibrillogenesis. The amino-terminal 70-kDa fragment contains assembly and gelatin-binding domains and is routinely used in FN binding and matrix assembly studies.