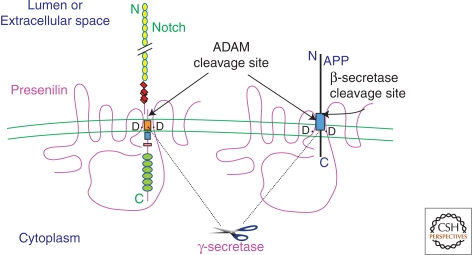
Figure 3.
Model of the role of presenilin (PS) in Notch and APP processing based on current information. Polytopic PS protein, which occurs principally as a cleaved heterodimer. Some Notch and APP molecules form complexes with PS. Two aspartates (D) in TM6 and TM7 of PS are required for the cleavages of Notch and APP within their TM domains, and these align with the respective sites of cleavage in the two substrates. PS-mediated proteolysis of both Notch and APP is preceded by ectodomain shedding by an ADAM family protease (“α-secretase”). Alternatively, APP can undergo ectodomain shedding by β-secretase. Several motifs are depicted in Notch: EGF-like repeats (yellow circles), LNG repeats (red diamonds), a single TM (orange box), the RAM23 domain (blue square), a nuclear localization sequence (pink rectangle), and six cdc10/ankyrin repeats (green ovals). Following the putative intramembranous cleavage mediated by PS, the Notch intracellular domain is released to the nucleus to activate transcription of target genes. APP contains the Aβ region (light blue box), which is released into the lumen after sequential cleavages of APP by β-secretase and then γ-secretase/PS. The APP intracellular domain is released into the cytoplasm.