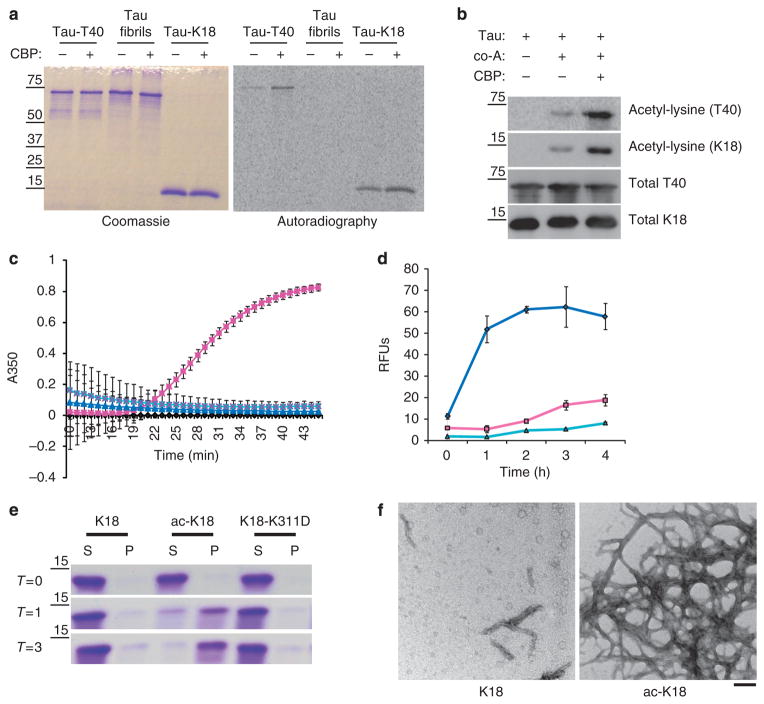
Figure 1. Tau acetylation impairs MT assembly and promotes tau fibrillization in vitro.
(a) Recombinant full-length T40, 4R-tau MT-binding domain K18, or tau fibrils (see Methods for fibrillization assay) were acetylated by incubation with [14C]-labeled acetyl-CoA in the presence or absence of CBP. Reaction products were analysed by SDS–PAGE and Coomassie blue staining followed by overnight radiographic exposure using STORM phosphor-imager software. (b) T40 or K18 proteins were incubated with acetyl-CoA and/or recombinant CBP and reaction products were subjected to immunoblot analysis using a polyclonal anti-tau-specific antibody for MT repeat domain (E10) and an anti-acetyl-lysine antibody. (c) MT assembly activities of K18 proteins were evaluated in light-scattering assays. Tubulin monomers (30 μM) were mixed with 40 μM tau proteins in MT assembly buffer supplemented with 2 mM guanosine triphosphate. MT assembly was determined by monitoring absorbance every minute at 350 nm using a SpectraMax plate reader. Colour key is as follows: native unmodified K18 (pink squares), acetylated K18 (blue triangles), K18 containing K311D mutation (turquoise X’s) and no tau control (black diamonds). (d) Tau proteins (10 μM) were evaluated in fibrillization reactions using 10 μM heparin to induce assembly. At each time point, samples were incubated with 12.5 μM ThT and excitation/emission wavelengths were set to 450 and 510 nm, respectively. Colour key is as follows: native unmodified K18 (pink squares), acetylated K18 (blue diamonds) and K18-containing K311D mutation (turquoise triangles). Both MT assembly and fibrillization reactions were confirmed from n = 4 independent experiments. Error bars indicate standard error of the mean. (e) Tau proteins at indicated fibrillization time points (T = 0, 1, 3 h) were centrifuged at 100,000 g for 30 min to generate a pellet fraction (P) containing tau fibrils and supernatants (S) containing unassembled tau protein. Samples were analysed by SDS–PAGE and Coomassie staining to monitor fibril formation. (f) Four-hour time points from fibrillization reactions were analysed by negative-staining EM. Note, at the concentration of 10 μM, native K18 did not fibrillize, whereas acetylated K18 had fibrillized extensively with morphologies similar to AD-like PHFs. Scale bar, 200 nm.