Table 1.
Deallylative Primary Amidine Formation.
| entry | amines [5.0 equiv]a | amidine products | yield [%]b |
|---|---|---|---|
| 1 |
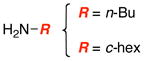
|
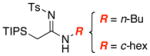 11 1112 |
87 |
| 2 | 92 | ||
| 3 |
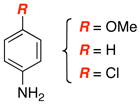
|
14 15 |
50 |
| 4 | 71 | ||
| 5 | 22 | ||
| 6 |
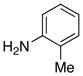
|
 16 16
|
30 |
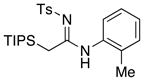
| 7 |
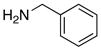
|
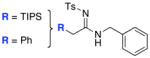 17 1718 |
≥95 |
| 8 | ≥95 |
All entries utilized ynamide 8a except in entry 8, Ph-substituted ynamide 8b was used. All reactions employed 5.0 mol % PdCl2(PPh3)2, 1.0 equiv K2CO3, THF [conc = 0.05 M], 80 °C, 5–8 h.
Isolated yields.
