Table 2.
Secondary Amidine Formation.
| entry | ynamides | aminesa | amidine products | yield [%]b |
|---|---|---|---|---|
| 1 |
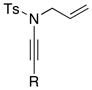 8a 8a8c 8d 8e |
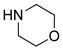
|
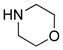
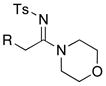 19: R = TIPS 19: R = TIPS20: R = t-Bu 21: R = (CH2)3OTBS 22: R = 2-MeO-Ph |
≥95 |
| 2 | ≥95 | |||
| 3 | 92 | |||
| 4 | 37 | |||
| 5 |
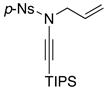 8f 8f
|
|
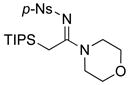 23 23
|
39 |
| 6 |
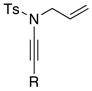 8a 8a8b 8g |
|
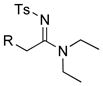 24: R = TIPS 24: R = TIPS25: R = Ph 26: R = n-hex |
≥95 |
| 7 | ≥95 | |||
| 8 | ≥95 | |||
| 9 |
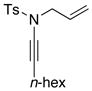 8g 8g
|
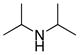
|
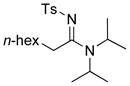 27 27
|
70 |
| 10 |
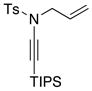 8a 8a
|
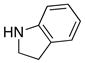
|
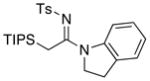 28 28
|
≥95 |
| 11 |
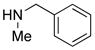
|
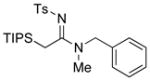 29 29
|
77 | |
| 12 |
|
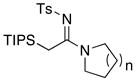 30a: n = 1 30a: n = 130b: n = 2 30c: n = 3 |
≥95 | |
| 13 | ≥95 | |||
| 14 | ≥95 | |||
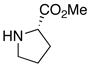
| 15 |
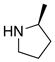
|
 31 31
|
91 | |
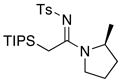
| 16 |
|
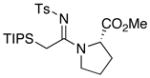 32 32
|
≥95 | |
| 17 |
|
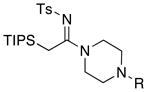 33a: R = CH3 33a: R = CH333b: R = Ph |
≥95 | |
| 18 | ≥95 | |||
| 19 |
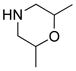
|
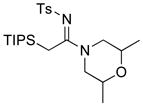 34 34
|
≥95 | |
| 20 |
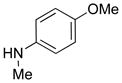
|
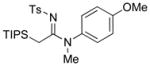 35 35
|
82 |
All reactions utilized 5.0 equiv of the amine, 5.0 mol % PdCl2(PPh3)2, and 1.0 equiv K2CO3; and were run in THF [conc = 0.05 M] at 80 °C over 5–8 h.
Isolated yields.
