Table 4.
Vinylogous Amidine Synthesis.
| entry | ynamides | enaminesa | vinylogous amidines | yield [%]b |
|---|---|---|---|---|
| 1 |
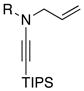 8a: R = Ts 8a: R = Ts8h: R = MBS |
|
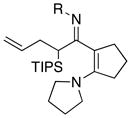 45 4546 |
52c |
| 2 | 71d | |||
| 3 |
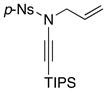 8f 8f
|
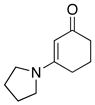 42 42
|
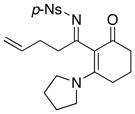 47 47
|
58e |
| 4 |
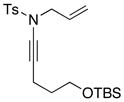 8d 8d
|
|
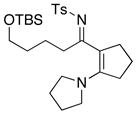 48 48
|
54f |
| 5 |
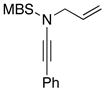 8i 8i
|
|
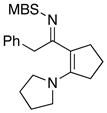 49 49
|
57g |
| 6 |
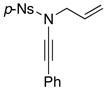 8j 8j
|
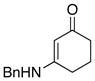 43 43
|
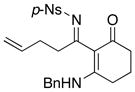 50 50
|
-- |
| 7 |
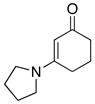 42 42
|
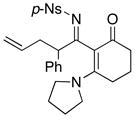 51 51
|
62h |
Unless otherwise noted, all reactions utilized 3.0 equiv of the enamine, 5.0 mol% Pd2(dba)3, and 10.0 mol% of xantphos; and were run in THF [conc = 0.05 M].
Isolated yields.
2 h at 70°C.
2 h at 50 °C with 1.5 equiv of K2CO3.
12 h at 70°C with 1.5 equiv of 42; 2.0 mol % Pd2(dba)3; and 4.0 mol% of xantphos.
30 min at 50 °C.
2 h at RT.
2 h at 75 °C with 1.5 equiv of K2CO3 and 1.5 equiv of 44.
